Content of this guide
This user guide is designed to assist new and existing users in navigating and utilizing the SPEAR reporting tool effectively. Its comprehensive coverage of all the features and functions of the platform will help you get the most out of SPEAR, making it easier for you create, submit and edit reports. Whether you are a beginner or an advanced user, this guide will provide you with a complete understanding of the SPEAR reporting tool, ensuring that you have the information you need to use it to its fullest potential.
List of content
1. Getting started
Accessing the Important to remember
- You can click the WMDA SPEAR reporting tool
To access the SPEAR reporting tool, simply type "https://spear.wmda.info" into your browser's address bar. To make it easier to get to the login page, you can save a bookmark in your browser.
If this is your first time accessing the SPEAR tool, you will need to create a password. Click on the "Forgot Password" button marked in red in figure 1. Fill out your email address (be careful, it's case-sensitive) on the password reset page in figure 2 and click the "Send Password Reset Email" button. You'll receive an email with a link to set a new password. Note that the password reset link will expire after 2 hours, so if you don't use it in time, you'll need to start the process again.
Setting and resetting your password
Step 1: Click on the 'Forgot Password' button on the SPEAR login screen (figure 1).Step 2: In the next screen, enter your e-mail address and click on the 'Send Reset Password Email' button.- logo on the top left of your screen to go back to the reports overview
- If you have an incident that requires quick dissemination as a rapid alert in our community, please contact the WMDA office directly by sending an e-mail at sear-spear@wmda.info and by phone on: +31 (0)88 505 7900.
- Data submitted via the SPEAR reporting tool must be limited to only the data elements that are required for reporting. SPEAR reporters recognize the importance of protecting donor and patient privacy and will not share identifiable data to maintain the confidence and trust of donors, patients and regulators.
- When using the tool, you agree to the WMDA Terms of Use and Privacy Policy
1. Getting started
To access the SPEAR reporting tool, simply type "https://spear.wmda.info" into your browser's address bar. To make it easier to get to the login page, you can save a bookmark in your browser.
If this is your first time accessing the SPEAR tool, you will need to create a password. Click on the "Forgot Password" button marked in red in figure 1. Fill out your email address (be careful, it's case-sensitive) on the password reset page in figure 2 and click the "Send Password Reset Email" button. You'll receive an email with a link to set a new password. Note that the password reset link will expire after 2 hours, so if you don't use it in time, you'll need to start the process again.
1.1 Setting (first time!) and resetting your password
- Step 1: Click on the 'Forgot Password' button on the SPEAR login screen (figure 1).
- Step 2: In the next screen, enter your e-mail address and click on the 'Send Reset Password Email' button.
| Note |
|---|
| title | Password reset link expires |
|---|
|
Please note: the link to reset your password expires in 2 hours. If you don't use it within that time, you must request a new password again by following the steps 1-2. |
| Imagefloat |
|---|
| caption | Figure 1: SPEAR login panel |
|---|
|
 Image Added Image Added |
1.2 Choosing roles
If you have multiple roles available to you (e.g. you are a SPEAR reporter and a SPEAR committee member or you are a SPEAR reporter for multiple organisations) then every time you login to the SPEAR reporting tool you have to select the role you initially want to access the tool with.
| Info |
|---|
|
Many users will only have 1 role (reporter) assigned to them. If this is you, you will not see the role selection panel after login, but instead you will automatically be directed to your report overview. You can skip this section of the user guide. |
- Step 1: Login to the SPEAR reporting with your username and password (see above).
- Step 2: If you have multiple roles available to you, you will now see a "role selection" panel below the login fields (figure 2)
- Step 3: Click on the role you wish to log in with at this time and click select. You are now directed to the report overview available to that role.
| Imagefloat |
|---|
| caption | Figure 2: Access menu to switch roles |
|---|
|
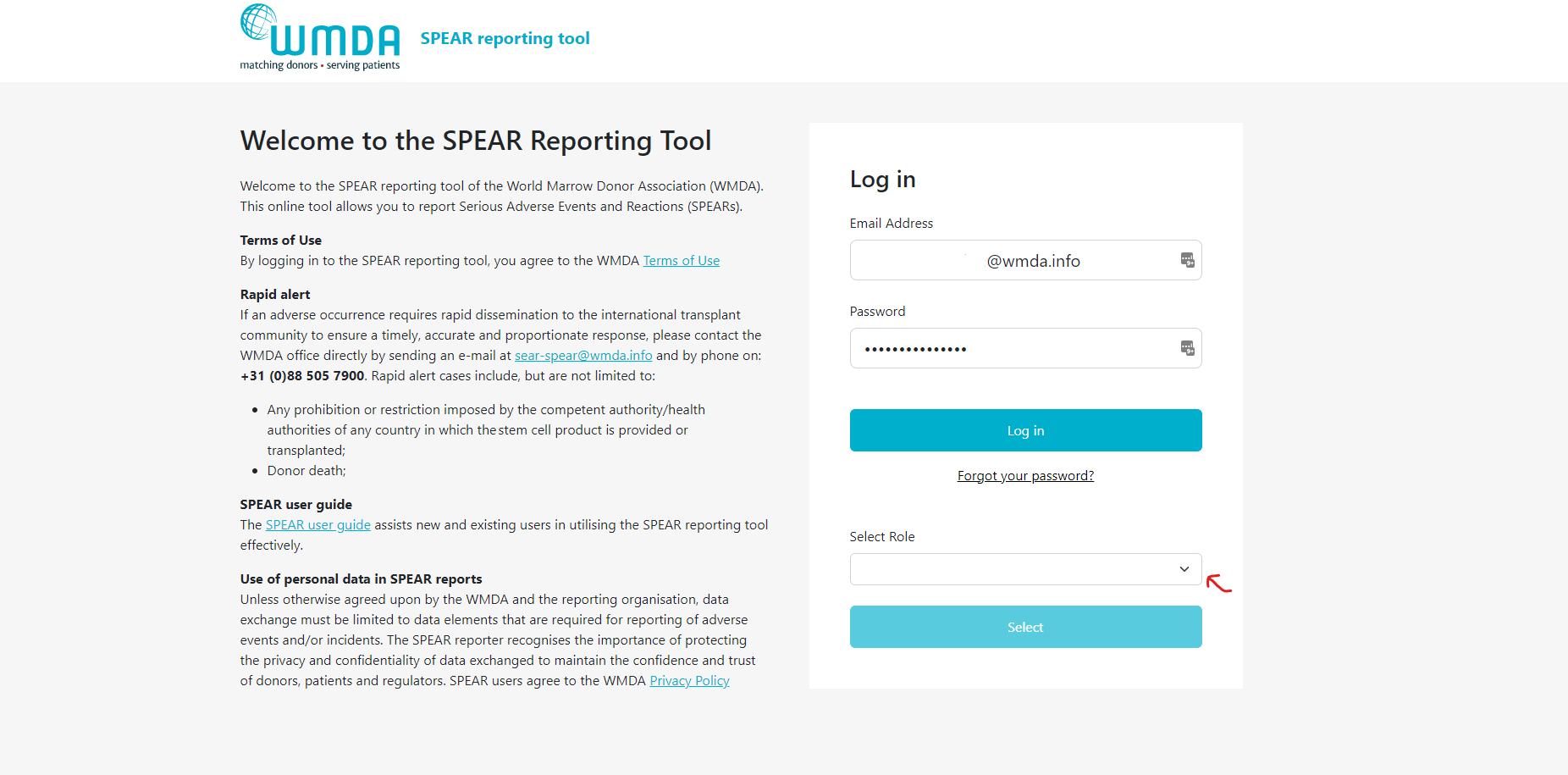 Image Added Image Added
|
1.3 Choosing roles when already logged in
If you have multiple roles available to you,
| Note |
|---|
| title | Password reset link expires |
|---|
|
Please note: the link to reset your password expires in 2 hours. If you don't use it within that time, you must request a new password again by following the steps 1-2. |
| Imagefloat |
|---|
| caption | Figure 1: SPEAR login panel |
|---|
|
 Image Removed Image Removed
|
Switching roles
Every time you login to the SPEAR reporting tool, you select the role you initially want to access the tool with. If you have multiple roles available to you (e.g. you are a SPEAR reporter and a SPEAR committee member or you are a SPEAR reporter for multiple organisations), then you can easily switch roles without logging out. The steps below explain how.
- Step 1: Login to the SPEAR reporting with your username and password (see above). Step 2: Click on the three dots next to your name on the top right side of the page (figure 2). A menu will appear that has the Switch Role option At the top of the page, on the right, you find your personal menu (figure 3). Click on your name to expand it.
- Step 32: Click this Switch Role option (figure 34) and you will be redirected to a new page where you can select the roles available to you.
- Step 43: Select Confirm the selection of the role you want and click by clicking the Select button . To confirm the role switch, a message will appear (figure 5). You will be automatically re-directed to the report overview where a message appears: "Successfully switched role to XX at YY". You can now use the SPEAR reporting tool in the role you selected. Click on the SPEAR logo at the left top corner of the page to go to the dashboard overview.you selected.
| Imagefloat |
|---|
| caption | Figure 23: Access Personal menu to switch roles |
|---|
|
 Image Removed Image Removed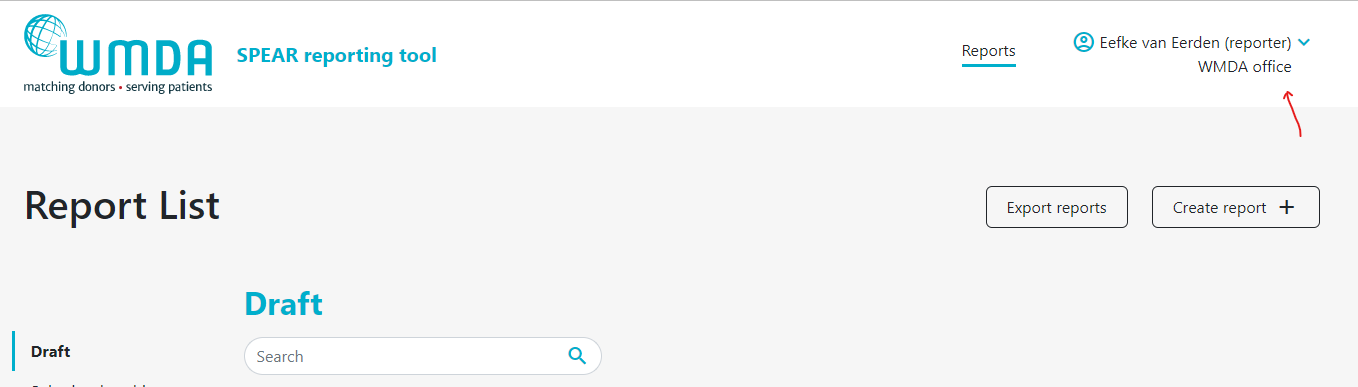 Image Added Image Added
|
| Imagefloat |
|---|
| caption | Figure 34: Select switch roles |
|---|
|
 Image Removed Image Removed
|
|
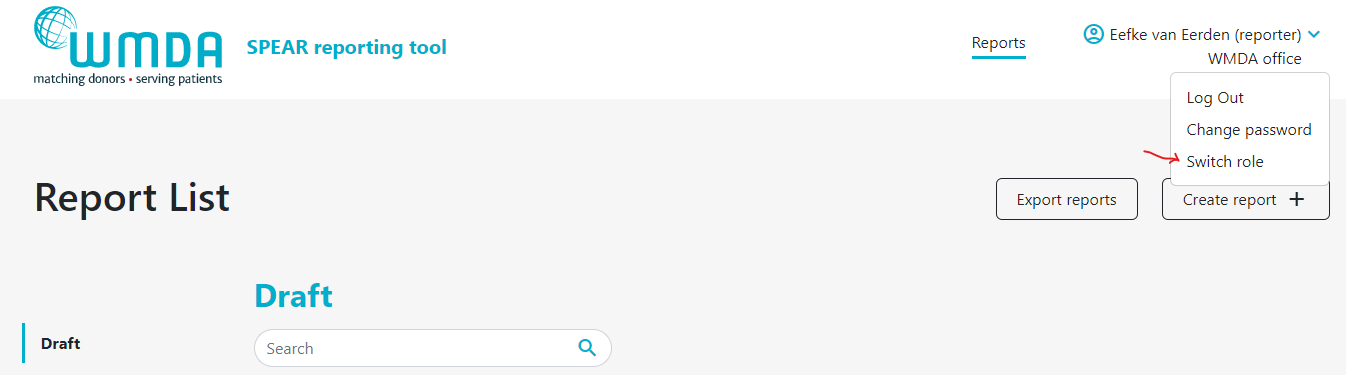 Image Added Image Added
|
| Imagefloat |
|---|
| caption | Figure 5: Panel to select role |
|---|
|
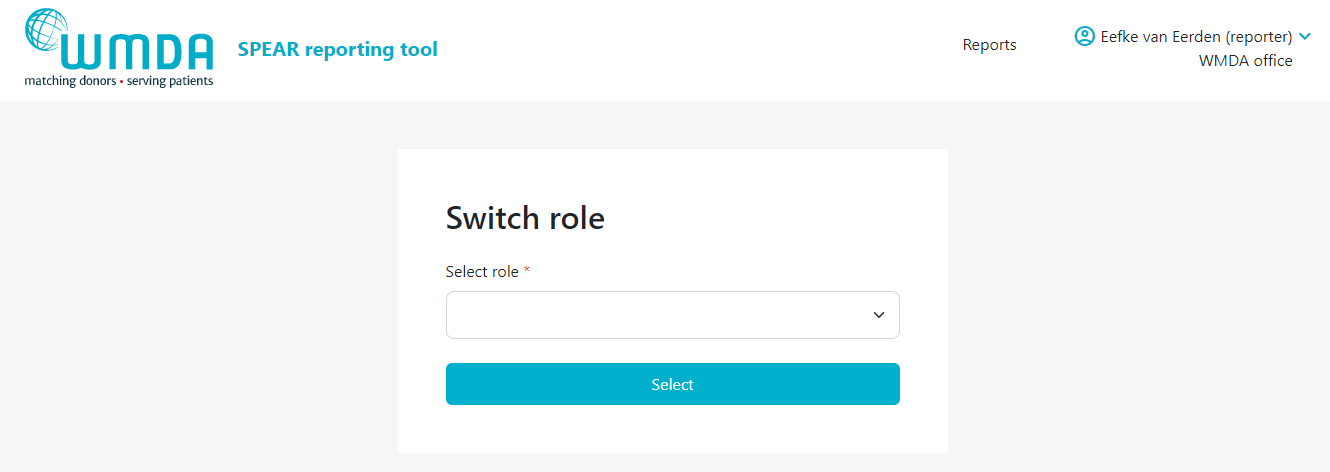 Image Added Image Added
|
1.4 Logging off
To close and exit the application click on the three dots next to your name on your personal menu at the top right side of the page (figure 43). A menu will appear that has the Logout option (figure 56). After logging out of the system, you will be redirected to the login screen.
| Imagefloat |
|---|
| caption | Figure 4: Access menu to log out |
|---|
|
 Image Removed Image Removed
|
| Imagefloat |
|---|
| caption | Figure 5: Select log out |
|---|
|
|
 Image Added Image Added Image Removed Image Removed
|
The SPEAR reporting tool is used for three main goals: 1) as a platform for reporters to submit possible SPEAR cases, 2) as a tool for the SPEAR committee to assess and review reports that are submitted and 3) to provide a central current database of adverse events and reactions for data analysis and research purposes. This is why there are a variety of roles available within the platform. The role you have (selected) determines the amount of data and detail of data you can view via the tool and the actions you can carry outand/or edit.
Drafting a report (reporter)
- Step 1: Login to the SPEAR online reporting tool with your username and password (see chapter 1). Don't have a username and password an account yet? Please see below at the FAQ: "How do I register as a SPEAR reporter?"
- Step 2: Choose the role of reporter with the correct organisation when you login to draft reportscreate reports (see chapter 1.2)
- Step 3: Click the Create new report button at the top of the page under Report list to start a new draft draft (figure 7).
Step 4: Start your new draft report by entering the Type of report first. Once you've selected the correct type of report, the rest of
the the form fields (or tabs) will
become be filled with questions based on the type of report you selected. Depending on the type of report selected, fields are available or not and can be mandatory (or not).
| Note |
|---|
| title | Change the report type |
|---|
|
You can always change the Type of report by going back to the first tab (Type of report). If you change the type of report selected, different follow-up questions might appear. Answers previously given can be automatically erased if they no longer are part of the question list belonging to the new |
Type of report you selected. Adding an attachment to your report (reporters)
Step 1: If you haven't done so yet, start by selecting a Type of report at the Type of report you selected. |
| Imagefloat |
|---|
| caption | Figure 7: Creating a report |
|---|
|
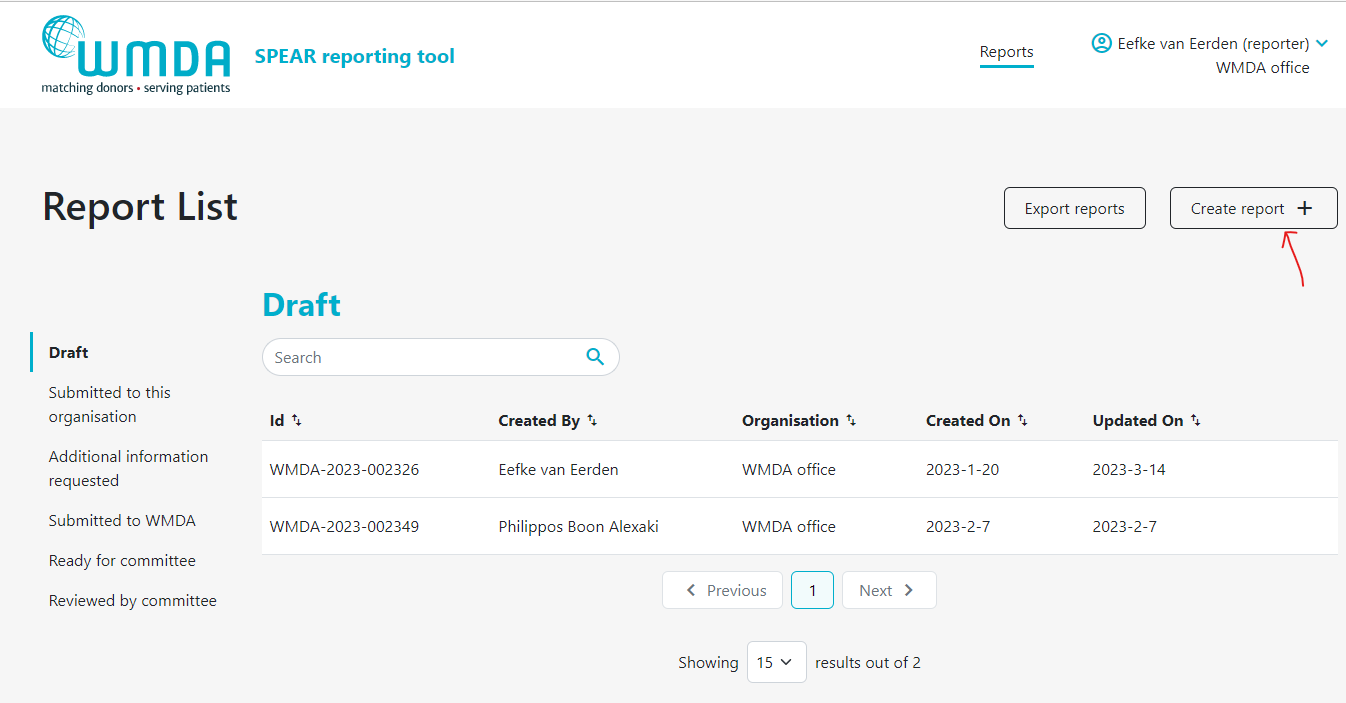 Image Added Image Added
|
Adding an attachment to your report (reporters)
- Step 1: Open the report you want to attach the file to by 1) opening an existing report or 2) creating a new draft report.
- Step 2: Go to the Finalisation tab (figure 8)
- Step 3: Locate the text "Upload any documents...." (figure 9)
Step 4: Click the Upload files button to select the files to upload from your computer. You can select multiple files from the same folder at once by using CTRL + click or by dragging your cursor
- Step 2: Go to the Finalisation tab
- Step 3: Scroll down to "Upload any documents.."
Step 4: Click the paperclip icon to select the files to upload, you can select multiple files from the same folder at once by using CTRL + click or by dragging your cursor
Note| title | File types and sizes |
|---|
Please note: only file with xlsx, .xls, .docx, .doc .pdf, .jpeg, .png, .gif, .tiff, .bmp, .txt extensions are accepted. Each file uploaded may not exceed 10mb. The maximum number of attachments may not exceed 5.Step 5: After selecting the files you wish to upload, you must check the checkbox (figure 10) underneath the file overview to state that you are not uploading any identifying personal information, such as names, dates of births or any other identifying information belonging to donors, patients or staff.
| Warning |
|---|
For every file you upload, you have to check the checkbox stating that the files you are uploading do not contain any personal information. Personal data are any information which are related to an identified or identifiable natural person. The data subjects are identifiable if they can be directly or indirectly identified, especially by reference to an identifier such as a name, an identification number, location data, etc. In practice, these also include all data which are or can be assigned to a person in any kind of way. For example, the telephone, credit card or personnel number of a person, account data, number plate, appearance, customer number or address are all personal data. |
For more information on personal data according to the GDPR, please click here. For more information on personal data according to the GDPR, please click here. |
| Imagefloat |
|---|
| caption | Figure 8: the finalisation tab |
|---|
|
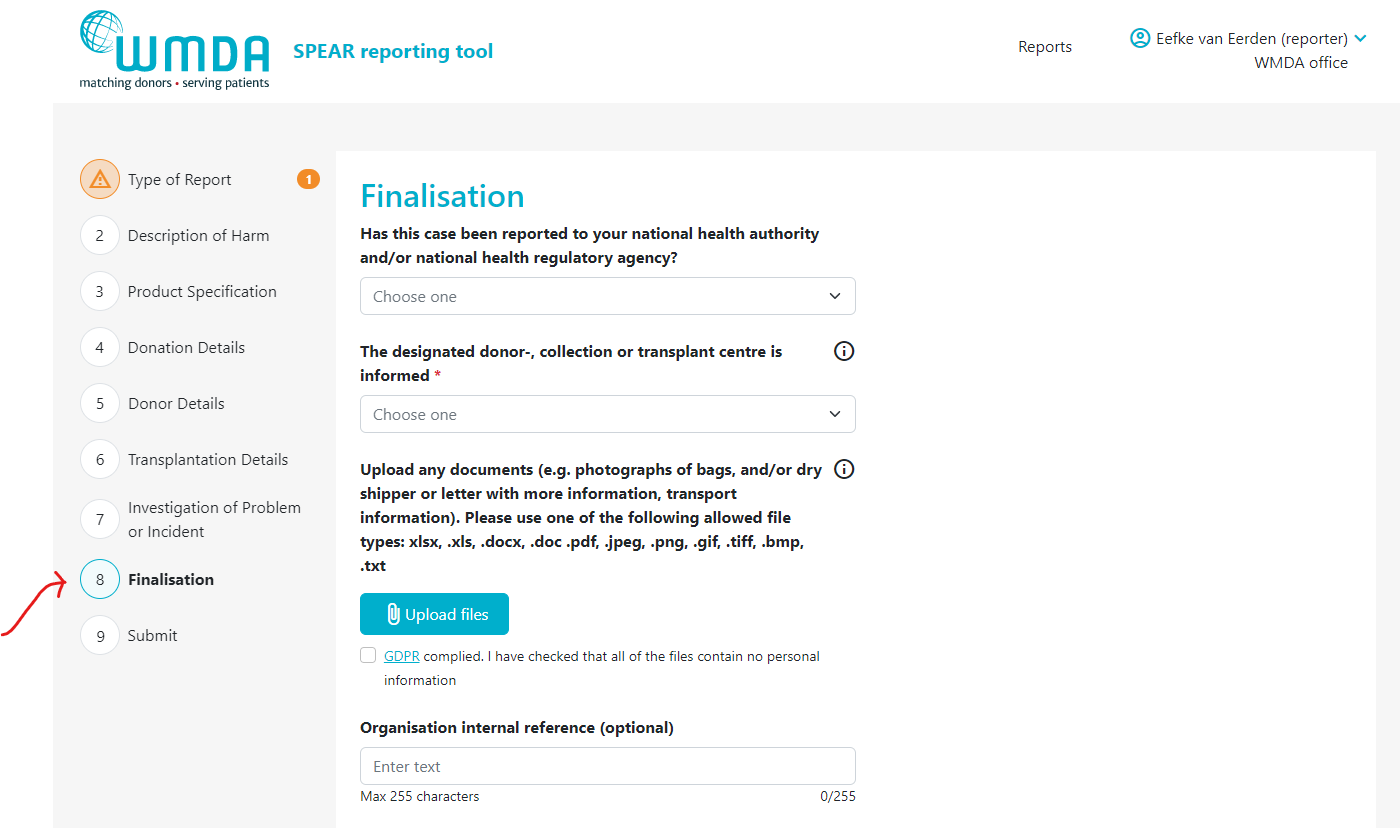 Image Added Image Added
|
| Imagefloat |
|---|
| caption | Figure 9: the Upload files button |
|---|
|
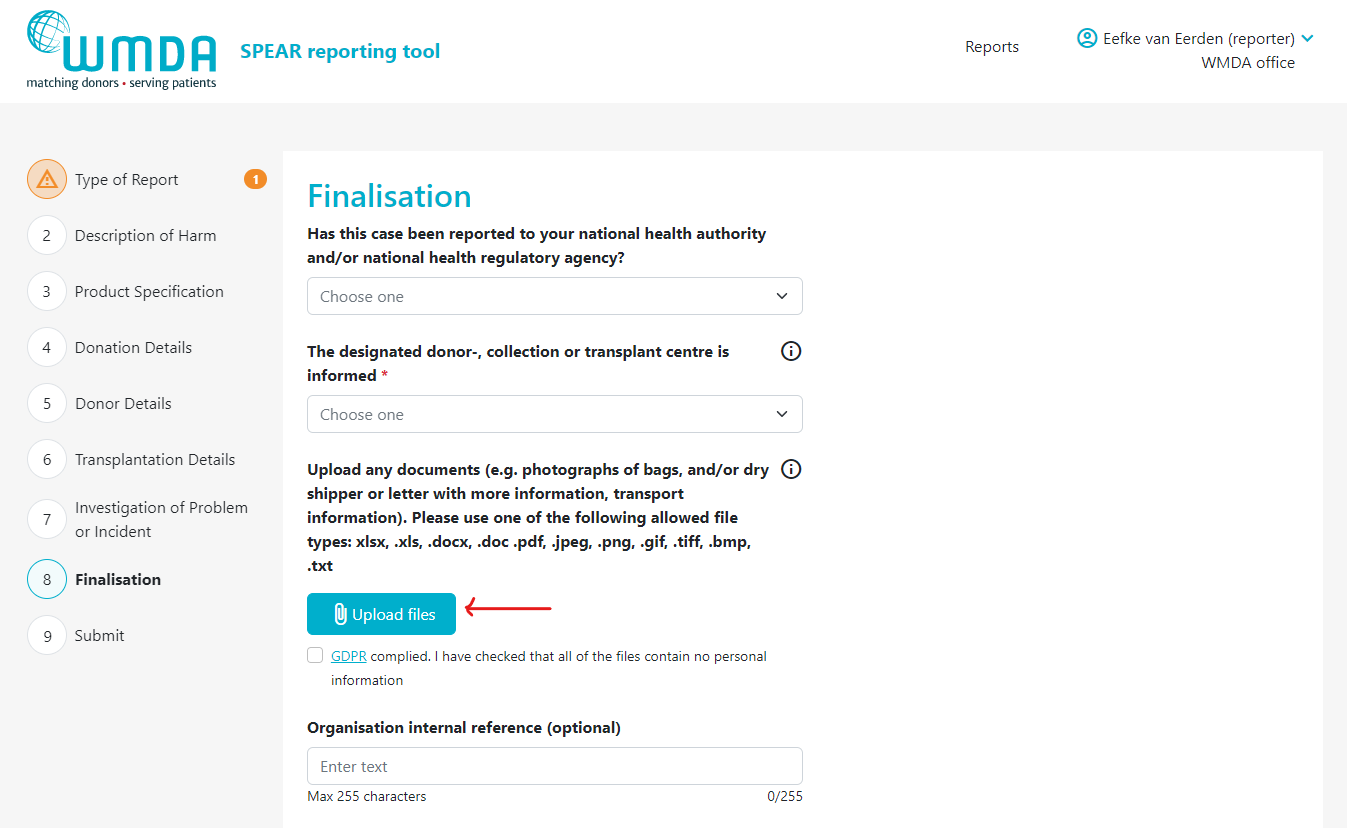 Image Added Image Added
|
| Imagefloat |
|---|
| caption | Figure 10: GDPR compliance |
|---|
|
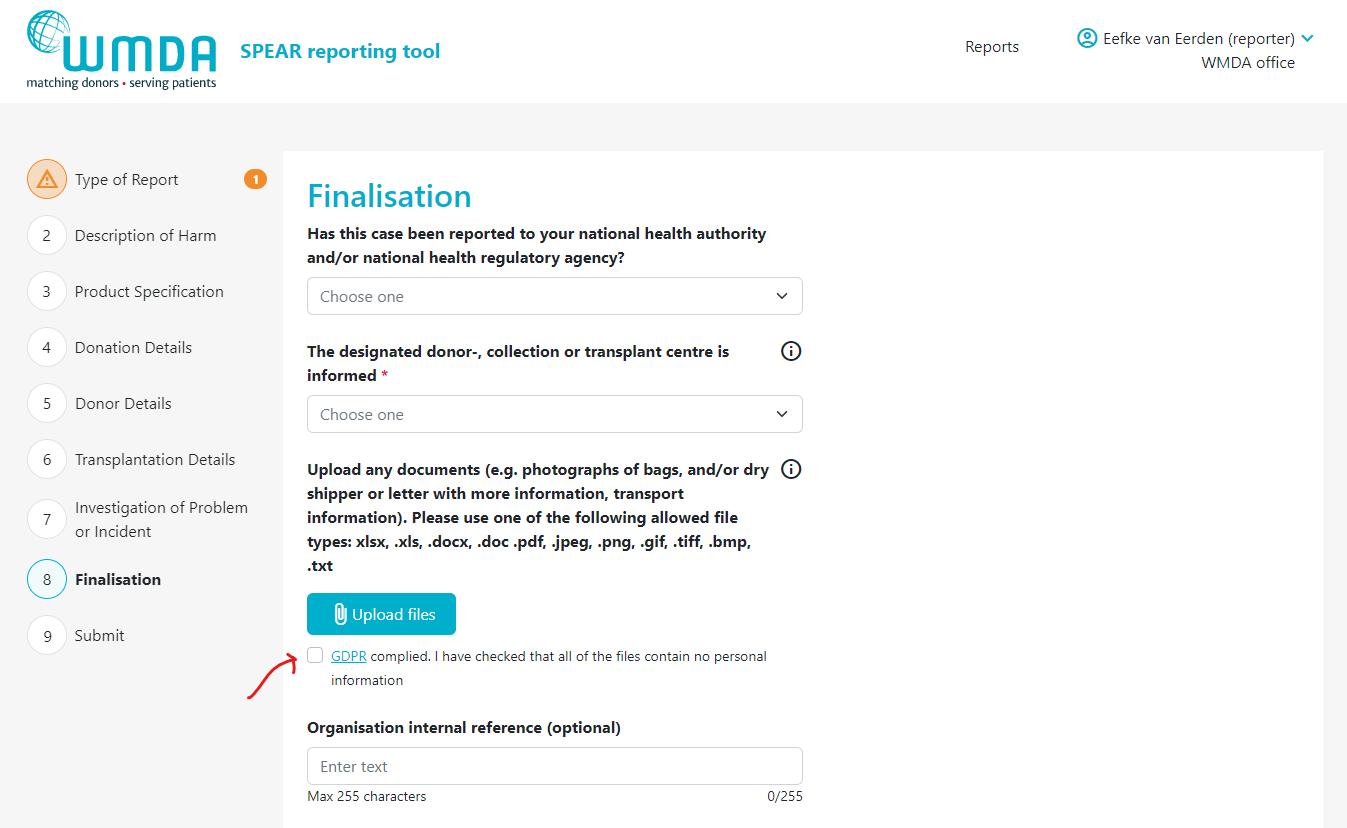 Image Added Image Added
|
Submitting a report (reporters)
Once you've filled out all required information in the report tabs, you are ready to submit the report. The last tab, labeled "Submit", gives a summary of what you've entered. If anything is missing, it will say so. If you see "This question is mandatory," go back to the indicated tab and answer the question. Otherwise, you won't be able to submit the report. If the summary page doesn't show any missing information, scroll down and click "Submit report." Depending on your organization type and your role, the report will be sent directly to WMDA or to your parent organization first.
Receiving a report from an affiliated (child) organisation (reporters)
If your organization has affiliated organizations, eg. a national donor registry with local transplant or collection centres, it could be that you receive reports from these organizations to submit to the WMDA on their behalf. If so, they are listed in a separate table on your dashboard. Please review the reports there in a timely manner. You can proceed to submit to the WMDA by opening the report in "view" mode and click the "submit to WMDA" button at the bottom of the page.
If you've submitted your report, it could be that the WMDA Medical advisor or SPEAR Committee has an additional question or a request for more details. Usually, this request follows within a few weeks after submitting the report to the WMDA. Sometimes it can be after a much longer time due to new discoveries or new similar cases being submitted. To add the requested information simply follow the steps below.
1. Log in to the
SPEAR Online Reporting Tool 2. Find the report in the
“Additional Info Requested” table.
- Click the Eye Icon to view the report OR
- Click the Pencil Icon to make changes to the report. Click “Submit to WMDA” on the final tab to submit the report again to the WMDA.
- To add comments, click the Text Bubble icon.
Providing updates to a report that has already been reviewed by the committee (reporters)
As long as a report has not been marked as "reviewed by committee", there is the possibility to add information via the SPEAR tool. You can click add comment or edit report to do so. If a report has already been reviewed, but you have crucial information to add, please email sear-spear@wmda.info to request to add the details to the report.
Review process of the committee (committee member)
Download report overview (all users)
You can download an overview of all reports in the SPEAR system of your organisation. Two file formats are provided for download: .csv (Comma Separated Value) file and .json (Javascript Object Notation) file. For regular use, we recommend choosing the .csv file for download as it is easily converted to an Excel file. In case you want to use the SPEAR data for IT applications: JSON is supported by all the programming languages like Java, Net (C#), PHP, etc.
JSON is a data exchange format that stands for JavaScript Object Notation with the extension .json. JSON is known as a light-weight data format type and is favored for its human readability and nesting features. It is often used in conjunction with APIs and data configuration.
CSV is a data storage format that stands for Comma Separated Values with the extension .csv. CSV files store data values (plain text) in a list format separated by commas. Notably, CSV files tend to be smaller in size and can be opened in text editors.
| Multimedia |
|---|
| name | SPEAR_download_export_instructions (1).mp4 |
|---|
| width | 50% |
|---|
| height | 50% |
|---|
|
Please follow the steps below to download an overview of reports:
- Step 1: When you are logged in to the correct role in SPEAR, scroll to the bottom of the page to find the Export reports button (figure 6)
- Step 2: In the pop up window, select if you want to download all reports or only the reports of a certain status (e.g. reviewed by committee) (figure 7).
- Step 3: In the pop up window, select the file format you'd like to download (figure 7)
- Step 3: Click download. You will be sent back to the dashboard report overview where you can see the progress of the download.
| Warning |
|---|
| title | Download might take a long time |
|---|
|
Currently downloading a .csv file will take a long time. Please note that it feature does work, it will just require a long wait time. Please go grab a cup of coffee and check back in a few minutes if the download is complete |
Converting a .CSV file to an .xslx (Excel) file:
- This two minute video explains how to open a .csv file in Microsoft Excel so you can use the data as a regular Excel file.
| Imagefloat |
|---|
| caption | Figure 6: Export reports button |
|---|
|

|
| Imagefloat |
|---|
| caption | Figure 7: Select JSON or CSV download |
|---|
|

|
Print a report (all users)
When you click the View option to open a report and view its details, a button will appear at the top of the page: "Print report".
Frequently Asked Questions (FAQ)
GENERAL
| Expand |
|---|
| title | Definition of adverse events |
|---|
|
The WMDA follows the EU definitions of a serious adverse event or reaction: 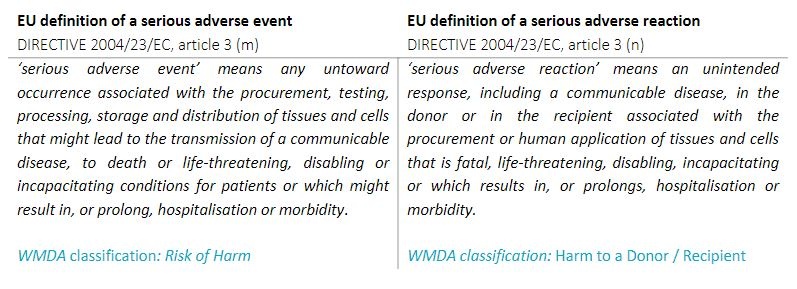
Definition serious adverse reaction (SAR): An unintended response, including a communicable disease, in the donor or in the recipient associated with the procurement or human application of tissues and cells that is fatal, life threatening, disabling, incapacitating or which results in, or prolongs, hospitalisation or morbidity. Definition serious adverse event (SAE): Any untoward occurrence associated with the procurement, storage and distrubution of tissues and cells that might lead to the transmission of a communicable disease, to death or life-greatening, disabling or incapacitating conditions for donors or patients or which might result in, or prolong, hospitalisation or morbidity. Criteria for serious adverse event (SAE): - The event (could have) led to inappropriate use of tissues or cells
- The event (could have) resulted in loss of the complete or significant quantity of irreplaceable cells
- The event (could have ) led to a serious adverse reaction (grade 2, 3 or 4)
- The event (could have) led to misidentification or an unintended switch of cells
- The event (could have) led to (an unforeseen) transmission of disease from donor to recipient
|
| Expand |
|---|
| title | Role definitions and process flow |
|---|
|
Donor, transplant or collection centres are affiliated with donor registries have the possibility to create reports and submit them to the reporting organisation/donor registry. The further handling of the report will go via the donor registry. The donor registry can approve and submit reports from submitting organisations as well as their own incident reports. When submitted, all reports are available to the WMDA office and the SPEAR Committee. The WMDA office team for SPEAR reporting consists of a medical advisor and a project coordinator. The medical advisor reviews all reported incidents at least once a week and checks for rapid alerts. If necessary, the medical advisor can request the reporting organization for additional information. If the report is approved by the medical advisor, it will be turned over to the SPEAR Committee. |
| Expand |
|---|
| title | How do I register as a SPEAR reporter? |
|---|
|
To become a SPEAR reporter, you contact the WMDA office by e-mail: sear-spear@wmda.info In this email please provide us with the following information: - First and last name of the SPEAR reporter
- Name of the organization the reporter will submit SPEARs for
- Type of organization:
- DR = Donor registry
- CBB = Cord blood bank
- DRCBB = Donor registry with CBU
- TC = Transplant centre
- DC = Donor Centre
- If applicable: if there's an affiliated parent organization, please provide the name of this organization. Please note that a parent organization can request access for their affiliated centres too.
|
| Expand |
|---|
| title | Can I share my account with colleagues? |
|---|
|
No, SPEAR accounts are intended for use by one person. A shared account would mean a shared password and this brings numerous security risks along with it. For example, if an employee leaves the organisation, they would still have access to highly sensitive SPEAR data because the username and password are known to them. Sharing passwords in any way, via email, post its, etc., should of course always be avoided at any cost as it creates the opportunity for an unwanted third party to gain access.
We do not mind creating multiple accounts, it actually makes it easier to manage if someone leaves or joins your organisation. SPEAR users from the same organisation also all have access to the same report data (e.g. draft reports can be started by 1 user and finished by another). |
USING THE SPEAR REPORTING TOOL
SPEAR REPORTING 101
| Expand |
|---|
| title | What to report and what not to report? |
|---|
|
WMDA member organisations and their affiliated organisations are obliged to report to WMDA any unexpected donor and/or patient issue or product quality issue in a timely manner. If necessary, urgent measures can then be implemented to protect donors and/or patients, such as a recall of one or more defective batch(es) from the market or change in policies and procedures. Any adverse event or reaction, or risk thereof, that occurs during any step in the stem cell donation process can and should be reported in the SPEAR online reporting tool. This includes adverse events or reactions that occur to patients and donors, related and unrelated. |
| Expand |
|---|
| title | How do I assess the imputability? |
|---|
|
The WMDA has developed an imputability assessment tool to help you determine the imputability grading most fitting with your adverse reaction report. |
| Expand |
|---|
| title | What ICD-10 refence can I use? |
|---|
|
Please make sure you use the correct ICD-10 code when prompted. You can refer to the ICD-10 database here. This will help us when we use the SPEAR data for short- or long-term analysis and to gain insight into possible trends. |
| Expand |
|---|
| title | Is this mobilising agent a Filgrastim, Lenograstim or other? |
|---|
|
Please see page 15 in this publication by IQVIA for a summary of EMA information for approved indications for G-CSF productsproducts |
| Expand |
|---|
| title | Reporting a Rapid Alert |
|---|
|
The aim of the Rapid Alert System is to transmit only those cases whose urgency and seriousness cannot permit any delay in transmission. For example, in |
| Expand |
|---|
| title | Reporting a Rapid Alert |
|---|
|
In case of recall or if there's a big risks risk for future donations or transfusions, a report can be submitted as a rapid alert. The rapid alert system ensures a timely, proportionate, accurate and consistent response to adverse occurrences arising from donation or transplantation which represent a significant threat to the international transplant community. This includes, but is not limited to: - any prohibition or restriction imposed by the competent authority/health authorities of any country in which the stem cell product is provided or transplanted;
- donor death;
If you have a rapid alert case to report, please submit it via the online reporting tool and contact the WMDA immediately by telephone (+31-(0)88 5057900) or e-mail at sear-spear@wmda.info If the report qualifies as a rapid alert and for dissemination to the international community, it will always be shared without any traceable information on the reporting agency, donor, patient or reporter. In addition, reporters should notify their competent authorities/health where incident has occurred. |
CASE STUDIES
to be updated















