Versions Compared
compared with
Key
- This line was added.
- This line was removed.
- Formatting was changed.
Serious (Product) Events and Adverse Reactions: S(P)EAR
The WMDA has set up a central global reporting system for WMDA member organisations to report Serious (Product) Events and Adverse Reactions – S(P)EARs – to the WMDA.
Aim
- To gain insight in the occurrence of serious events and adverse effects in relation to blood stem cell donation by unrelated donors and blood stem cell collection/processing from unrelated donors.
Purpose
To collect and analyze information on recipient and donor serious adverse events (SAE) and reactions which affect donors and/or products from all WMDA stem cell
donor registries and cord blood banks.To follow a rapid alert system for disseminating information on SAE/R to all members of the international community in contact with allogeneic donors and patients.
Quick links
- Submit a SEAR/SPEAR incident
- Examples of SEAR/SPEAR reports
- Common Terminology Criteria for Adverse Events (CTCAE) v5.0
- International Statistical Classification of Diseases (ICD) 10th revision
- Notify Library
- Imputability Assessment Tool
- Operating of the S(P)EAR Committee
- Standard Operating Procedure for reporting SEAR/SPEAR
| Info | ||
|---|---|---|
| ||
S(P)EAR annual reports |
| Info | ||
|---|---|---|
| ||
S(P)EAR rapid alerts |
| Info | ||||
|---|---|---|---|---|
| ||||
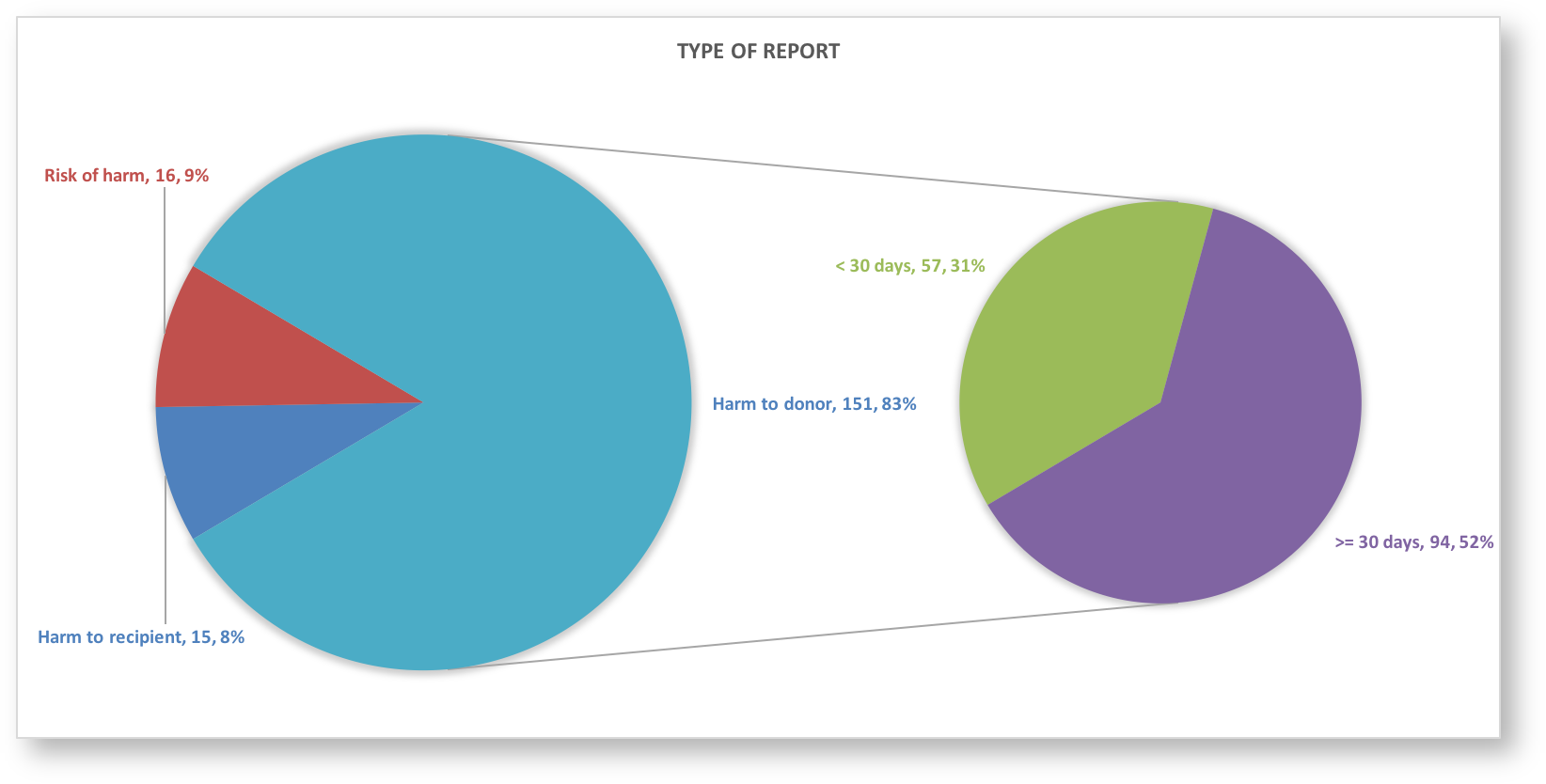
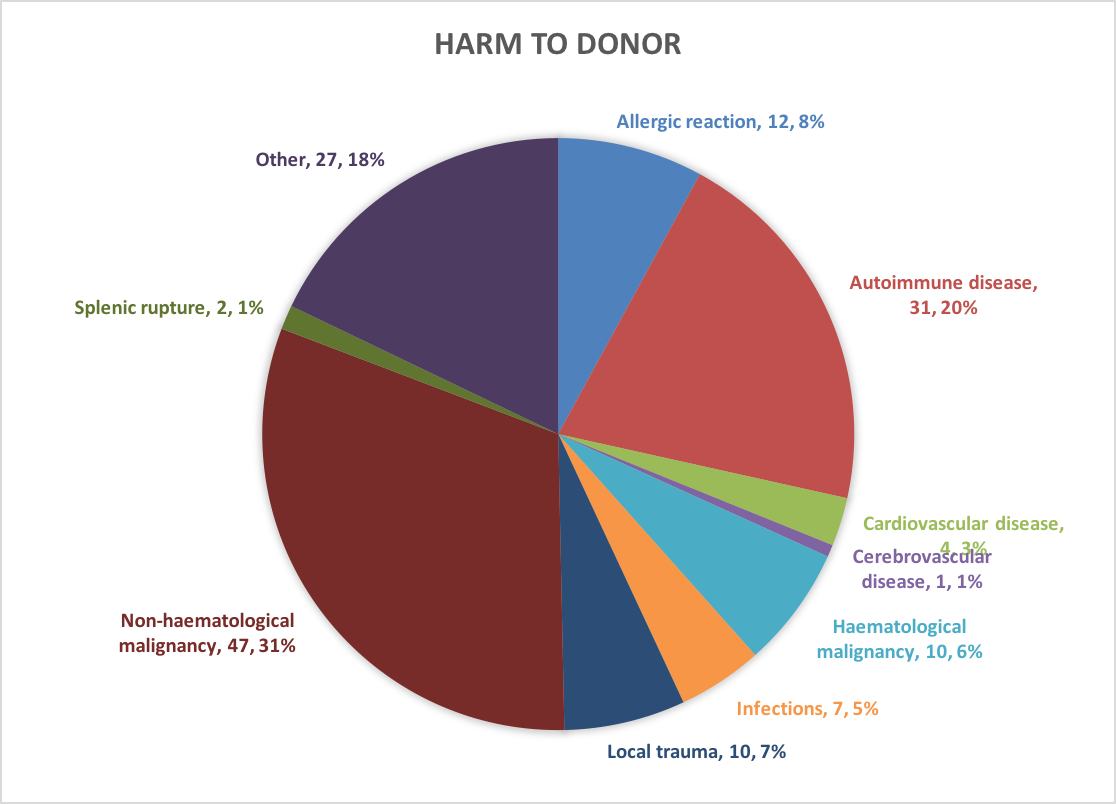
See for more graphs the S(P)EAR Annual Report of 2018 |