...
Questions are divided into the following subjects:
//insert content overview
GENERAL
| Expand |
|---|
| title | I forgot my username/password. What should I do? |
|---|
|
When you go to the SPEAR reporting tool login page you will find the login module where you can enter your email address and password. Click on “forgot password?” button underneath the Password field. Enter your email address in the designated field and press the “Submit” button. You will then receive a link to reset your password. If you haven't received the email with a reset link for your password within 24 hours (please check your spam folder too), please contact the WMDA office at sear-spear@wmda.info |
...
| Expand |
|---|
| title | Definition of adverse events |
|---|
|
The WMDA follows the EU definitions of a serious adverse event or reaction: 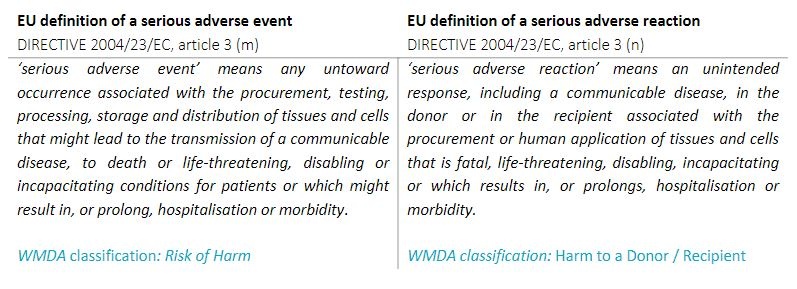
|
2022 REBUILD OF USING THE SPEAR REPORTING TOOL
| Expand |
|---|
| title | What new features are available in the 2022 rebuild of SPEAR? |
|---|
|
|
SPEAR REPORTING 101
| Expand |
|---|
| title | What to report and what not to report? |
|---|
|
WMDA member organisations and their affiliated organisations are obliged to report to WMDA any unexpected donor and/or patient issue or product quality issue in a timely manner. If necessary, urgent measures can then be implemented to protect donors and/or patients, such as a recall of one or more defective batch(es) from the market or change in policies and procedures. Any adverse event or reaction, or risk thereof, that occurs during any step in the stem cell donation process can and should be reported in the SPEAR online reporting tool. This includes adverse events or reactions that occur to patients and donors, related and unrelated. |
...
| Expand |
|---|
| title | What ICD-10 refence can I use? |
|---|
|
|
| Expand |
|---|
| title | Reporting a Rapid Alert |
|---|
|
In case of recall or big risks for future donations or transfusions a report can be submitted as a rapid alert. This includes, but is not limited to: - any prohibition or restriction imposed by the competent authority/health authorities of any country in which the stem cell product is provided or transplanted;
- donor death;
Please flag your report as a rapid alert after submitting it by contacting WMDA the by telephone (+31-(0)88 5057900) or e-mail (sear-spear@wmda.info). If the report qualifies as a rapid alert and for dissemination to the international community, it will always be shared without any traceable information on the reporting agency, donor, patient or reporter. In addition, reporters should notify their competent authorities/health where incident has occurred. |
CASE STUDIES