CBB Survey 2015
These data are submitted by the cord blood bank to the WMDA in February 2015. The WMDA makes no representations or express or implied warranties regarding any information on this site or obtained through these links; expressly disclaims all legal liability and responsibility for the accuracy, completeness, currency, suitability, validity, or usefulness of such information; and is not and will not be liable for any statements, errors, or omissions in posted information, or for any losses, injuries, or damages that arise or are alleged to arise from such information. Use of any information provided on this site does not and is not intended to create a contractual or other relationship. All information is on an as-is basis. The data cannot be updated.
| 1. General Information |
|---|
| Name of CBB | DKMS Cord Blood Bank |
| CBB Director | Alexander |
| CBB Director | Platz |
| Address | Enderstraße 94, Building C |
| Address | 01277 |
| Address | Dresden |
| Phone Number | +49-351-2509660 |
| Website | www.dkms-nabelschnurblutbank.de |
| Date CBB Started Collecting Cord Blood Units (month/day/year) | 01/01/1997 |
| Number of Public Cord Blood Units | 8,428 |
| Planned Number of Public Cord Blood Units Stored in 2015 | 500 |
| Lists on BMDW | Yes |
| Affiliated with National Stem Cell Donor Registry | Yes |
| Registry Affiliation | ZKRD Zentrales Knochenmarkspender-Register für die Bundesrepublik Deutschland gemeinnützige GmbH |
| 2. Cord Blood Units in Inventory |
| Current Processing Method |
| Volume reduction with SEPAX | |
| Year Current Process Method Started | 1997 |
| Percent of Units Plasma and RBC Reduced (manual) | 0 |
| Percent of Units Plasma and RBC Reduced (automated) | 100 |
| Percent of Units RBC Reduced Only | 0 |
| Percent of Units No Volume Reduction | 0 |
| 3. Accreditations, Licenses and Certifications |
|---|
| FACT-Netcord | Yes |
| AABB | No |
| Competent Authority/ National Health Authority | Yes |
| Name of Competent Authority | Landesdirektion Sachsen + Paul-Ehrlich-Institut |
| Audited by National Stem Cell Registry | Yes |
| ISO | No |
| 4. Cord Blood Collection |
| Current Collection Practice Is the collection In/Ex -Utero or both? | In-utero |
| Current Antiseptic | Alcohol |
| Collection Bag | Single needle |
| Agitation during Collection | Manual |
| 5. Conditioning/Transport from Collection Site to CBB |
| Secondary Bag Used | Yes |
| Transport Conditions | Qualified transporter |
| Transport Conditions | Insulating transport container |
| Transport Conditions | Passive refrigeration system |
| Transport Conditions | Electronic temperature probe |
| Transport Conditions | Ground transport |
| Temp. for Storage and Transport | Room temperature |
| 6. Pre-Processing Evaluation |
| Completed Prior to Accepting a CBU | Medical history, collection report, informed consent |
| Current Threshold for Accepting a CBU | Viability threshold CD45 |
| Method for CD34 Remuneration | ISHAGE guidelines |
| External Proficiency Testing for QC of FACS Lab | Yes, other |
| External Proficiency Testing for QC of FACS Lab | Instand e.V., RV No. 217 |
| Time from Collection to Processing | up to 48H |
| 7. Processing and Packaging |
| Pre Freeze Processing Methods- Unit in Inventory | SEPAX |
| Pre Freeze Processing Methods- Unit in Inventory | Other: Centrifugation + volume reduction |
| Pre Freeze Processing Methods- Current | SEPAX |
| Additives Currently in Use | No additive |
| Current Cryopreservation Method | Conventional CRF |
| Current Cryopreservation Method | Other: Icecube 14 M-B |
| Current Cryoprotectant Additive | DMSO |
| Current Cryobag | Single bag 80:20 |
| Current Target Cryopreservation Volume (mL) | 25.0 |
| Current Packaging for Storage | Canister |
| Current Packaging for Storage | More than one segment |
| 8. Testing |
| Extra Material Currently Stored | Cord blood material for DNA extraction |
| Extra Material Currently Stored | Plasma/cord blood |
| Extra Material Currently Stored | Maternal material for DNA extraction |
| Extra Material Currently Stored | Maternal plasma/serum |
| Current Post Processing Threshold for Accepting a CBU for Public Use TNC | 50 |
| Current Post Processing Threshold for Accepting a CBU for Public Use CD34 (10^6) Single Platform | 0,1 |
| Current Post Processing Threshold for Accepting a CBU for Public Use CD34 (10^6) Double Platform | NA |
| Current Post Processing Threshold for Accepting a CBU for Public Use CFU-GM | 1x10^4 |
| Current Post Processing Threshold for Accepting a CBU for Public Use CFU | 1x10^4 |
| Current Post Processing Threshold for Accepting a CBU for Public Use Viability | 85 |
| 9. Storage |
| Type of Storage Container Used | Conventional storage tank vapor phase |
| Monitoring of Storage | Centralized alarm system local |
| Monitoring of Storage | Centralized system remote monitoring |
| Monitoring of Storage | LN2 level |
| Monitoring of Storage | System default |
| Monitoring of Storage | Temperature monitoring |
| 10. HLA Typing |
| Current Level of HLA Typing at Time of Listing HLA-A | HR |
| Current Level of HLA Typing at Time of Listing HLA-B | HR |
| Current Level of HLA Typing at Time of Listing HLA-C | HR |
| Current Level of HLA Typing at Time of Listing HLA-DRB1 | HR |
| Current Level of HLA Typing at Time of Listing HLA-DQB1 | HR |
| Current Level of HLA Typing at Time of Listing HLA-DPB1 | HR |
| Accreditation of HLA Lab | ASHI accredited lab |
| Average Turnaround Time for Extended HLA Typing Results in days | 3 |
| Attached Segment Used for Confirmatory/ Verification Typing | Yes |
| Units Listed without Attached Segment and have not been Previously Typed on Attached Segment | No |
| Percentage of CBUs that have an Attached Segment |
| 100% | |
| Confirmatory/ Verification Typing on an Attached Segment is Pre-Release Requirement | Yes |
| 11. Reservation and Cancellation Policies |
|---|
| What Point is a CBU Reserved for a Patient | HLA typing request |
| What Point is a CBU Reserved for a Patient | Reservation request |
| What Point is a CBU Reserved for a Patient | Shipment request |
| Length of Time a CBU can be Reserved in days | 90 |
| Reservation Fee | No |
| Reservation Cancellation Fee in Absence of Shipment Request | No |
| Can Reservation be Extended | Yes |
| Is a Unit Report Provided on a Unit that is Reserved for Another Patient | No |
| Is TC Informed when CBU is Released | Yes |
| 12. Release and Shipment |
| Hemoglobinopathy Screening Performed Prior to Release | Yes |
| Criteria to Ship a CBU Viability and Cell Count |
| Viability 85% and TNC should meet minimum dose per kg/recipient´s body weight | |
| Criteria to Ship a CBU HLA Identity Testing | Yes |
| Current Packaging for Shipment to TC | Metal canister |
| Current Packaging for Shipment to TC | One attached segment |
| Current Packaging for Shipment to TC | Transport rack |
| Time Between Shipment Request and Sending CBU | 1-2 weeks |
| Fee for Shipment Cancellation | No |
| Dry Shippers Validated to Maintain Temperature of at least -150 for 48 hours Beyond Expected Arrival | Yes |
| Electronic Temperature Data Logger on All Dry Shippers | Yes |
| Who Selects Transport Company | CBB |
| Who Selects Transport Company | World Courier if the TC does not want other courier |
| Shape of Transport Container | Mushroom |
| 13. Adverse Events Reporting |
|---|
| Who are S(P)EARS Reported To Competent Authority | Yes |
| Who are S(P)EARS Reported To Internal Report | Yes |
| Who are S(P)EARS Reported To National Registry | Yes |
| Who are S(P)EARS Reported To Transplant Center | Yes |
| Who are S(P)EARS Reported To WMDA | Yes |
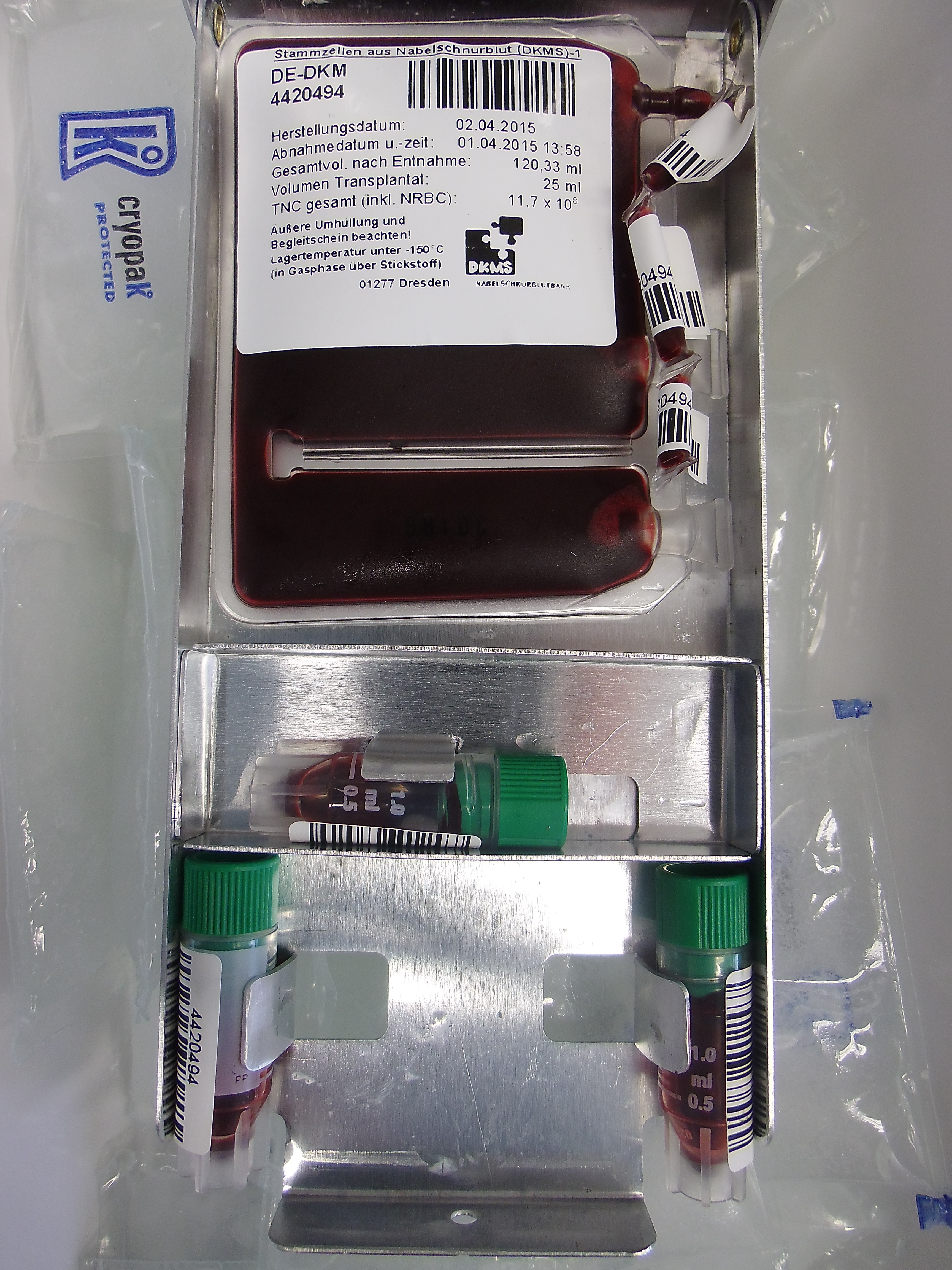
14. Pictures of cord blood units in the inventory
15. Infectious Disease Marker (IDM) CURRENTLY performed.
| View file | ||||
|---|---|---|---|---|
|
Attachments
...
Holiday Calendar
| Team Calendars | ||
|---|---|---|
|