#
XML file format
1. Introduction
The overall scope of the development phase two is to receive more data from our listing organisations and to make these data available through our Search & Match Service. However, the old format (DOT20) is not an appropriate format when you have many different fields/columns. Therefore, we had to move to another file format. The new file format is an XML (Extensible Markup Language) file, which is considered an industry standard that is extendable, robust and easy to use.
Several people from the community formed a working group to create the required XML Schema Definition (XSD) files. These files define the elements that are allowed in the XML file, the order of the elements and the values that will be accepted. The names of the elements are based upon EMDIS specifications and aligns with the EMDIS Data Dictionary when appropriate. Several elements are basic elements that should be included in all files, but there are also elements that are specific for only donors or only cord blood units (CBUs).
We will now explain the composition of the XML file and how you should use the XSD reference files.
2. XSD schema files
We provided two XSD schema files that define the structure of your XML file: basicTypes.xsd and Inventories.xsd.
The Inventories file describes the structure of the XML file and the order of the elements. Here you can also find if a certain field is mandatory or not (minOccurs="0"-> not mandatory). This file includes many "complexTypes" : an XML element that contains other elements and/or attributes. In the file you can see that the values of the elements can be defined here, like the elements GRID and ID, or that after the name of the field a "type" is defined. For example for the element with name BIRTH_DATE you see type="bareDateType". The definition of "bareDateType" is described in the basicTypes.xsd file.
We will now describe the global structure of the XML file and the elements.
Please note: For a lot of elements, we use abbreviations as allowed values. The explanation of all those abbreviations can be found in the XSD files. Most abbreviations are also the same as used for EMDIS and clarified in the EMDIS dictionary.
2.1 InventoryType elements
Field Identifier | Required | Description | Type | Length | Comment |
|---|---|---|---|---|---|
| CREATION_TIME | Yes | Creation time stamp of the inventories (in UTC) | dateTime | minimal 20 | Without fractional seconds the length is 20, for example: 2016-08-23T13:16:48Z. Additional notes: CREATION_TIME is defined as "Creation time stamp of the <INVENTORIES>" that means the time in UTC when the complete and valid file was finally created at the registry. This can be the same as SNAPSHOT_TIME. |
| LISTING_ORGANIZATION | Yes | Organisation that lists the donor/cbu provided as ION | ionType: number between 1000 and 9999 | 4 | Issuing Organisation Number (ION) allocated by ICBBA. This can be different from the POOL when another organisation is sending the data to WMDA. |
| POOL | Yes | Physical location of the donors/CBUs of the inventory provided as ION | ionType: number between 1000 and 9999 | 4 | Physical location of the donors/CBUs of the inventory provided as ION. |
| CONTENT_TYPE | Yes | Type of the inventory items, i.e. donor ("D") or CBU ("C") | contentTypeType | 1 | The content-type is also shown in the fileName. When CONTENT_TYPE is "D", the INVENTORY must contain <DONOR>-blocks. When CONTENT_TYPE is "C", the INVENTORY must contain <CBU>-blocks. |
| UPDATE_MODE | Yes | Update mode of the inventory, i.e. FULL or DIFF | updateModeType | 4 | Only UPDATE_MODE "FULL" is currently supported. Always the complete inventory should be send. |
| SNAPSHOT_TIME | No | Timestamp of the 'data snapshot' (in UTC) | dateTime | minimal 20 | Without fractional seconds the length is 20, for example: 2016-08-23T13:16:48Z Additional notes: SNAPSHOT_TIME in the element <INVENTORY> is defined as "timestamp of the data snapshot in UTC" that means the timestamp of the creation of this part of the complete file. This can be the timestamp of the XML export and I guess that in most of the cases it will be identical to the CREATION_TIME. |
| SCHEMA_VERSION | Yes | Version of the applied XML Schema Definition (XSD) | schemaVersionType | The schema version is very important as this determines the validation rules that should be applied during the processing of your file. |
2.2 ItemBaseType elements (for Donors and CBUs)
Field Identifier | Required | Description | Type | Length | Comment |
|---|---|---|---|---|---|
| ID | Yes | Unique identifier of the donor/CBU | String | 17 | Unique identifier of the donor/CBU: If you are an EMDIS member, you can use the same ID as you use for that system (EMDIS hub code + donor identification allocated by the associated donor registry). For non-EMDIS members we recommend to use two digit ISO country code of the associated donor registry + donor identification allocated by the associated donor registry. For example: AU600196166, DEGOE-35487, US087013165, SB45. However, you are also allowed to use just the donor ID allocated by your registry. |
| GRID | No | Global registration identifier of the donor | String | 19 | ONLY applicable for donors. GRID format allowed is: XXXXXXXXXXXXXXXXXXX. Only upper case letter and numbers are allowed. The first 4 characters include the ION were the donor is registered. The following 13 characters contain the donor specific ID and the last two characters are check characters that are calculated from the 17 previous characters. |
| ATTR | No | Describing attribute of the donor/CBU according to house rules of the sending organization. | String | 3 | |
| BIRTH_DATE | Yes | Date of birth of the donor/CBU | bareDateType | 10 | Date without timezone information, example 1968-06-28, Date Delimiter = "-" |
| SEX | No | Biological gender of the donor/CBU | sexType | 1 | sexType: "F","M" F = Female M = Male NOTE: Mandatory for donors, optional for CBUs |
| ABO | No | Blood group (ABO) of the donor/CBU | aboType | 2 | aboType: "A","B","O","AB" |
| RHESUS | No | Rhesus (Rh) factor of the donor/CBU | rhesusType | 1 | rhesusType: "P","N"
P = Positive NOTE: "+" and "-" are not supported |
| ETHN | No | Ethnic group of the donor/CBU | ethnType | 4 | ethnType: "AFNA","AFSS", "ASSW", "ASSO", "ASCE", "ASSE", "ASNE", "ASOC", "CAEU", AFNA = African: North Africa |
| CCR5 | No | CCR5 status of the donor/CBU | ccr5Type | 2 | ccr5Type: "DD","WW","DW" DD = Deletion (delta 32) - homozygous |
| HLA | Yes | HLA of the donor/cbu | hlaType | Explained separately at hlaType 2.3 | |
| KIR | No | KIR genotype of the donor/CBU | kirType | Explained separately at kirType 2.4 | |
| IDM | No | Infectious disease markers (IDM) and other relevant tests of the donor/CBU | idmType | Explained separately at idmType 2.5 | |
| RSV_PAT | No | Unique identifier of the patient the donor/CBU is reserved for (STATUS=RS). | String | 17 | The value comprises the EMDIS patient identification, where the patient search centre is an EMDIS member, otherwise the value is empty. For example: AU9654021, DE275342, US2277450. NOTE: This field is not required for status "RS" and can be transmitted as empty if privacy concerns exist. |
| STATUS | Yes | Status of the donor/CBU | statusType | 2 | statusType: "AV" ,"TU" ,"RS" ("DE" is not supported yet) AV = Available for transplantation purposes |
| STAT_END_DATE | No | Date until which the current status will be applicable | bareDateType | 10 | Date without timezone information, example 1968-06-28, Date Delimiter = "-" |
2.3 hlaType elements
HlaType fields can be divided in hlaSerFieldsType and hlaDnaFieldsType
hlaSerFieldsType: HLA values obtained by serological typing methods
hlaSerFieldsType = “<FIELD1>” string of max length 5 “</FIELD1>”, “<FIELD2>” string of max length 5 “</FIELD2>”;
Example: <SER><FIELD1>1</FIELD1><FIELD2>5</FIELD2></SER>
Serological typing results can be given for loci that are defined as hlaLocusType. These loci include HLA-A, -B, -C, -DRB1, -DQB1.
hlaDnaFieldsType: HLA values obtained by DNA based typing methods
hlaDnaFieldsType = “<FIELD1>” string of max length 20 “</FIELD1>”, “<FIELD2>” string of max length 20 “</FIELD2>”;
Exanple: <DNA><FIELD1>01:01</FIELD1><FIELD2>05:01</FIELD2></DNA>
DNA typing results can be given for loci that are defined as hlaLocusType and hlaLocusDnaOnlyType. These loci include HLA-A, -B, -C, -DRB1, -DQB1, -DRB3, -DRB4, -DRB5, -DQA1, -DPA1, -DPB1.
Finally, previously the dot20 file format allowed to submit values like 01 in DNA fields. We can no longer accept this and you have to submit the equivalent of 01, so '01:XX' .
Minimal required elements
Minimal typing values for Donor: A (either SER or DNA), B (either SER or DNA)
Minimal typing values for CBU: A (either SER or DNA), B (either SER or DNA), DRB1 (either SER or DNA)
Please note:
- It is no longer possible to submit string HLA values; only single values are allowed.
- When a donor or CBU has homozygous alleles/values, please use the following notation:
<HLA><A><SER><FIELD1>1</FIELD1><FIELD2 /></SER></A> ...
or
<DQB1><DNA><FIELD1>05:02:01G</FIELD1><FIELD2 /></DNA></DQB1>
Field Identifier | Required | Description | Type | Length | Comment |
|---|---|---|---|---|---|
| SER | depends on content type and DNA fields provided | HLA values obtained by serological typing methods | hlaSerFieldsType | 5 | Each SER element contains two other elements: FIELD1 and FIELD2 |
| DNA | depends on content type and SER fields provided | HLA values obtained by DNA based typing methods | hlaDnaFieldsType | 20 | Each DNA element contains two other elements: FIELD1 and FIELD2 |
| FIELD1 | HLA value of allele 1 | 5 or 20 | Element within the element SER and DNA | ||
| FIELD2 | HLA value of allele 2 | 5 or 20 | Element within the element SER and DNA | ||
| A | Yes | HLA-A values | hlaLocusType | Both SER and DNA possible; either SER or DNA values required | |
| B | Yes | HLA-B values | hlaLocusType | Both SER and DNA possible; either SER or DNA values required | |
| C | No | HLA-C values | hlaLocusType | Both SER and DNA possible | |
| DRB1 | Yes (CBU) No (Donor) | HLA-DRB1 values | hlaLocusType | Both SER and DNA possible; either SER or DNA values required for CBU | |
| DRB3 | No | HLA-DRB3 values | hlaLocusDnaOnlyType | Only DNA possible | |
| DRB4 | No | HLA-DRB4 values | hlaLocusDnaOnlyType | Only DNA possible | |
| DRB5 | No | HLA-DRB5 values | hlaLocusDnaOnlyType | Only DNA possible | |
| DQA1 | No | HLA-DQA1 values | hlaLocusDnaOnlyType | Only DNA possible | |
| DQB1 | No | HLA-DQB1 values | hlaLocusType | Both SER and DNA possible | |
| DPA1 | No | HLA-DPA1 values | hlaLocusDnaOnlyType | Only DNA possible | |
| DPB1 | No | HLA-DPB1 values | hlaLocusDnaOnlyType | Only DNA possible |
2.4 kirType elements
The kirType Field Definitions consists of the type: kirLocusType. This is defined as a String with 3 characters: "POS" or "NEG". "POS" means "Presence of KIR gene", "NEG" means "Absence of KIR gene".
The following elements are possible and in this specific order:
<KIR2DL1>,<KIR2DL2>,<KIR2DL3>,<KIR2DL4>,<KIR2DL5A>,<KIR2DL5B>,<KIR2DS1>,<KIR2DS2>,<KIR2DS3>,<KIR2DS4>,<KIR2DS5>,<KIR2DP1>,<KIR3DL1>,<KIR3DL2>,<KIR3DL3>,<KIR3DS1>,<KIR3DP1>.
There is another field called <KIR_GL> (URI that refers to a GL-string registered with a GL-service or direct GL-string for absence / presence) this field is not used at the moment and must be empty.
Field Identifier | Required | Description | Type | Length | Comment |
|---|---|---|---|---|---|
| KIR gene e.g. KIR2DL1 | No | KIR genotype e.g. KIR gene 2DL1 | kirLocusType | 3 | valid values: "POS" = presence of KIR gene; "NEG" = absence of KIR gene |
2.5 idmType elements
There are many infectious disease markers (IDM) possible in the element IDM. Many IDM elements can have either the values idmValueType or idmValueExtType
idmValueType includes the following values: "P","N"
idemValueExtType include the following values: “P”,“G”,“M”,“B”,“H”,“O”,“N”
Field Identifier | Required | Description | Type | Length | Comment |
|---|---|---|---|---|---|
| CMV | No | CMV status | idmValueExtType | 1 | idmValueExtType: “P”,“G”,“M”,“B”,“H”,“O”,“N” P = IgG or IgM positive, test did not differentiate EMDIS data dictionary also has a ‘Q’ (questionable / unclear) but that will not be applicable within the data submission file. |
| CMV_NAT | No | CMV NAT status | idmValueType | 1 | idmValueType: "P","N" P = Positive N = Negative |
| CMV_DATE | No | Date of CMV test | bareDateTyp | 10 | Date without timezone information, example 1968-06-28, Date Delimiter = "-" |
| HBS_AG | No | Hepatitis B status (hepatitis B surface antigen) | idmValueType | 1 | idmValueType: "P","N" P = Positive N = Negative |
| ANTI_HBC | No | Hepatitis B status (antibody to hepatitis B core antigen) | idmValueType | 1 | idmValueType: "P","N" P = Positive N = Negative |
| ANTI_HBS | No | Hepatitis B status (antibody to hepatitis B surface antigen) | idmValueType | 1 | idmValueType: "P","N" P = Positive N = Negative |
| ANTI_HCV | No | Hepatitis C status (antibody to hepatitis C virus) | idmValueType | 1 | idmValueType: "P","N" P = Positive N = Negative |
| ANTI_HIV_12 | No | Anti-HIV 1/2 status | idmValueType | 1 | idmValueType: "P","N" P = Positive N = Negative |
| HIV_1_NAT | No | HIV-1 NAT status | idmValueType | 1 | idmValueType: "P","N" P = Positive N = Negative |
| HIV_P24 | No | HIV p24 status | idmValueType | 1 | idmValueType: "P","N" P = Positive N = Negative |
| HCV_NAT | No | HCV NAT status | idmValueType | 1 | idmValueType: "P","N" P = Positive N = Negative |
| ANTI_HTLV | No | Antibody to HTLV I/II | idmValueType | 1 | idmValueType: "P","N" P = Positive N = Negative |
| SYPHILIS | No | Syphilis status | idmValueType | 1 | idmValueType: "P","N" P = Positive N = Negative |
| WNV | No | WNV status | idmValueType | 1 | idmValueType: "P","N" P = Positive N = Negative |
| CHAGAS | No | Chagas status | idmValueType | 1 | idmValueType: "P","N" P = Positive N = Negative |
| EBV | No | EBV status | idmValueExtType | 1 | idmValueExtType: “P”,“G”,“M”,“B”,“H”,“O”,“N” P = IgG or IgM positive, test did not differentiate EMDIS data dictionary also has a ‘Q’ (questionable / unclear) but that will not be applicable within the data submission file. Please leave blank for Q. |
| TOXO | No | Toxoplasmosis status | idmValueExtType | 1 | idmValueExtType: “P”,“G”,“M”,“B”,“H”,“O”,“N” P = IgG or IgM positive, test did not differentiate EMDIS data dictionary also has a ‘Q’ (questionable / unclear) but that will not be applicable within the data submission file. Please leave blank for Q. |
| HBV_NAT | No | HBV NAT status | idmValueType | 1 | idmValueType: "P","N" P = Positive N = Negative |
| PB19_NAT | No | ParvoB19 NAT status | idmValueType | 1 | idmValueType: "P","N" P = Positive N = Negative |
| ALT | No | Alanine aminotransferase status in units per litre | Short | Number, no decimals, minimal value is 1 |
2.6 donItemType elements
DonItemType elements contain elements that are specific for donors and not applicable for CBUs.
Field Identifier | Required | Description | Type | Length | Comment |
|---|---|---|---|---|---|
| STAT_REASON | No | Additional information relevant to the donor status | statReasonDonType | 2 | statReasonDonType: "DO", "DD","MR", "PR","TX", "MO", "UC", "OT", "TQ", "UK" DO = Donor is too old |
| CONTACT_DATE | No | Date of last confirmed contact - defined as the date of an active form of communication (e.g. a query about status, an address update, confirmation of their interest in donating) via any channel (e.g. email, mail, phone, website), post registration, from a donor to the registry. Any communication from the registry to the donor that does not lead to an activity of the donor suggesting his further interest in donation is explicitly excluded (e.g. annual mailing without reaction). | bareDateType | 10 | Date without timezone information, example 1968-06-28, Date Delimiter = "-" |
| CHECKUP_DATE | No | Date of the last medical checkup - defined as the date of a donor health assessment that indicates whether a donor is minimally suitable to be considered for donation, regardless if eligible for only one donation type, and includes questions about current medication and health issues (e.g. completion of a health screening questionnaire at Extended Typing or Verification Typing). The donor health assessment can be completed by any means (e.g. paper-based, online, phone). This does not require any physical examination of a donor. | bareDateType | 10 | Date without timezone information, example 1968-06-28, Date Delimiter = "-" |
| WEIGHT | No | Weight in kg | Short | Number between 1 and 999, no decimals | |
| HEIGHT | No | Height in cm | Short | Number between 1 and 999, no decimals | |
| NMBR_TRANS | No | Number of blood transfusions | Short | Number: zero or greater, no decimals | |
| NMBR_PREG | No | Number of pregnancies | Short | Number: zero or greater, no decimals | |
| NMBR_MARR | No | Number of marrow donations | Short | Number: zero or greater, no decimals | |
| NMBR_PBSC | No | Number of PBSC donations | Short | Number: zero or greater, no decimals | |
| COLL_TYPE | No | Collection type, i.e. the willingness of the donor to donate in a specific manner | String | 1 | collTypeType: "M", "P","B" M = Marrow |
2.7 cbuItemType elements
CbuItemType elements contain elements that are specific for CBUs and not applicable for donors.
Field Identifier | Required | Description | Type | Length | Comment |
|---|---|---|---|---|---|
| STAT_REASON | No | Additional information relevant to the CBU status | statReasonCbuType | 2 | statReasonCbuType: "QR","AD","CD","DS","XP","MR","OT","UK" Proposed reasons for Status TU:
|
| LOCAL_ID | No | Identification of CBU locally at the associated CBB | String | 17 | |
| BAG_ID | No | Identification as it appears on the bag. If more than one bag is available then this data attribute is not populated | String | 17 | |
| BANK_MANUF_ID | No | Unique identifier of the CBB that manufactured the CBU. ID shown in table in tab Cord blood bank IDs | String | 10 | PLEASE NOTE: For the upload the fields BANK_MANUF_ID and BANK_DISTRIB_ID should be fulfilled with a new ID for the corresponding cord blood banks and not with the EMDIS IDs. These IDs are important to allow WMDA to identify if the CBU is from an accredited bank which will be displayed within a search report. |
| BANK_DISTRIB_ID | No | Unique identifier of the CBB distributing the CBU. ID shown in table in tab Cord blood bank IDs | String | 10 | PLEASE NOTE: For the upload to WMDA the fields BANK_MANUF_ID and BANK_DISTRIB_ID should be fulfilled with a new ID for the corresponding cord blood banks and not with the EMDIS IDs. These IDs are important to allow WMDA to identify if the CBU is from an accredited bank which will be displayed within a search report. |
| COLL_DATE | No | Date that the CBU was collected | bareDateType | 10 | Date without timezone information, example 1968-06-28, Date Delimiter = "-" |
| PROC_DATE | No | Date that the processing started | bareDateType | 10 | Date without timezone information, example 1968-06-28, Date Delimiter = "-" |
| PROC_METH | No | Processing method used | procMethType | 3 | procMethType: "HES","DGS","CEN","FIL","FIC","PER","OTH" HES = Hydroxy-Ethyl-Starch NOTE: Values "NOT" and "UNK" are not supported "NOT" can now be found in CB_PROD_MOD = "NOT", "UNK" has to be transmitted as empty (CB_PROD_MOD = "") |
| PROC_METH_TYPE | No | Processing method type used | procMethTypeType | 3 | procMethTypeType: "MAN","SPX","OTP","AXP","OTH" MAN = Manual |
| FREEZE_DATE | No | Date that the CBU was frozen | bareDateType | 10 | Date without timezone information, example 1968-06-28, Date Delimiter = "-" |
| FREEZE_METH | No | Freezing method used | freezeMethType | 1 | freezeMethType: "C","M" C = Controlled Rate |
| PROD_MOD | No | Product modifications made | prodModType | 3 | prodModType: "BCE","DNE","PLR","PRR","RBR","NOT","OTH" BCE = Buffy Coat Enriched |
| BAG_TYPE | No | Type of bag used (bag fractions / split unit) | bbagTypeType | 5 | bagTypeType: "80/20","50/50","40/60","NS" (no split) |
| BAGS | No | Number of bags for CBU sub units | Short | Number between 1 and 99, no decimals | |
| BACT_CULT | No | Bacterial culture | cultValueType | 1 | cultValueType: "P","N","D" P = Positive N = Negative D = Not done |
| FUNG_CULT | No | Fungal culture | cultValueType | 1 | cultValueType: "P","N","D" P = Positive N = Negative D = Not done |
| HEMO_STATUS | No | Hemoglobinopathy screening status | hemoStatusType | 2 | hemoStatusType: "DN","DU","NS","CD","NC","DT","DD" DN = Screening done, normal results |
| VOL | No | Collected volume before processing (without additives) in ml | Short | Number between 1 and 9999, no decimals | |
| VOL_FRZN | No | Total volume frozen (post processing, prior to cryopreservation) in ml | Short | Number between 1 and 9999, no decimals | |
| TNC | No | Total number of nucleated cells (before processing) | Float | Number with decimals | |
| TNC_FRZN | No | Total number of nucleated cells (post processing, prior to cryopreservation) | Float | Number with decimals | |
| RED_BC_FRZN | No | Total number of nucleated red blood cells (post processing, prior to cryopreservation) | Float | Number with decimals: minimum is 0.0E0, maximum is 999.9E7 | |
| MNC_FRZN | No | Total Number of mononucleated cells (post processing, prior to cryopreservation) | Float | Number with decimals | |
| CD34PC | No | Total number of CD34+ cells (before processing) | Float | Number with decimals | |
| CD34PC_FRZN | No | Total number of CD34+ cells (post processing, prior to cryopreservation) | Float | Number with decimals | |
| CFU_FRZN | No | Total count of colony forming units (post processing, prior to cryopreservation) | Float | Number with decimals | |
| VIABILITY | No | Viability as percentage value | Short | Number between 0 and 100, no decimals | |
| VIABILITY_DATE | No | Date that viability was tested | bareDateType | 10 | Date without timezone information, example 1968-06-28, Date Delimiter = "-" |
| VIABILITY_CELLS | No | Type of cells tested for viability | viabilityCellsType | 6 | viabilityCellsType: "TNC","CD34PC","CD45PC" NOTE: VIABILITY_CELLS = "CD34PC" corresponds to CB_VIABILITY_CELLS = "CD34" in EMDIScord. VIABILITY_CELLS = "CD45PC" corresponds to CB_VIABILITY_CELLS = "CD45" in EMDIScord. |
| VIABILITY_METHOD | No | Method used to calculate the viability | viabilityMethodType | 2 | viabilityMethodType: "7A","PI","TB","OT" 7A = 7AAD |
| ATT_SEG | No | Number of attached segments available | Short | Number between 0 and 99, no decimals | |
| DNA_SMPL | No | DNA samples available? | Boolean | true,false | |
| OTH_SMPL | No | Samples other than DNA available? | Boolean | true,false | |
| CT_COMPLETE_DATE | No | Date of completion of confirmatory typing (CT) | bareDateType | 10 | Date without timezone information, example 1968-06-28, Date Delimiter = "-" |
| CT_SMPL_TYPE | No | Confirmatory typing (CT) sample type | ctSmplTypeType | 2 | ctSmplTypeType: "AS","WB","RC","FP","ED" AS = CBU Contiguous Attached Segment |
| AL_RED_BC | No | Number of red cell fraction aliquots | Short | Number between 0 and 99, no decimals | |
| AL_SER | No | Number of serum aliquots available | Short | Number between 0 and 99, no decimals | |
| SER_QUANT | No | Total quantity of serum available in ml | Float | Number between 0.0 and 99.9, one decimal | |
| AL_PLA | No | Number of plasma aliquots available | Short | Number between 0 and 99, no decimals | |
| PLA_QUANT | No | Total quantity of plasma available in ml | Float | Number between 0.0 and 99.9, one decimal | |
| MAT | No | Data of the mother of the infant associated with the CBU | matType | see further on this webpage matType |
2.8 matType elements
The matType elements are a sub-element from the element CBU.
Field Identifier | Required | Description | Type | Length | Comment |
|---|---|---|---|---|---|
| ID | No | Identification used to identify the maternal donor as assigned by the registry | String | 15 | |
| ID_BANK | No | Identification used by associated CBU manufacturer to identify maternal detail | String | 15 | |
| HLA | No | HLA of the mother of the infant associated with the CBU | hlaType | see above in section 2.3 hlaType | |
| IDM | No | Infectious disease markers (IDM) and other relevant tests of the mother of the CBU | idmType | see above in section 2.5 idmType | |
| AL_SER | No | Number of serum aliquots available | short | Number between 0 and 99, no decimals | |
| SER_QUANT | No | Total quantity of serum available in ml | Float | Number between 0.0 and 99.9, one decimal | |
| AL_PLA | No | Number of plasma aliquots available | Short | Number between 0 and 99, no decimals | |
| PLA_QUANT | No | Total quantity of plasma available in ml | Float | Number between 0.0 and 99.9, one decimal |
#
Minimal required data
Organisations providing donor or CBU data, should at least include the following elements with valid values. Without this data, the records will be rejected during the validation procedure.
A DONOR record should include:
- ID
- BIRTH_DATE
- SEX
- HLA (including at least HLA-A (SER or DNA) and HLA-B (SER or DNA))
- STATUS
A CBU record should include:
- ID
- BIRTH_DATE
- HLA (including at least HLA-A (SER or DNA), HLA-B (SER or DNA) and HLA-DRB1 (SER or DNA))
- STATUS
#
XML example files
We already provided you the XSD files, but these files do not show directly how an XML file with those definitions will look like. Therefore we created some example files: one for donors and one for CBUs.
Both files contain only 2 records, but in those two records almost all possible elements contain a value. It can help you to check the order of the elements in your own XML file. Please be aware that values like GRID are fictive and do not follow the rules for the check character. These two examples are based on the XSD files version 2.1.
Example donor file: ION-1234-D.xml
Example CBU file: ION-1234-C.xml
Validation of your XML files
After generating your XML files, you are advised to validate the generated XML file. You may use any XML tool that includes validation for that, for example the open source Qxmledit or XMLLINT.
You can use xmllint by invoking the program giving the XML file and the Schema file, and it will generate a report.
If the file validates, it will show:
xmllint --schema Inventories.xsd --huge --stream --sax --noout ION-1234-C.xml ION-1234-C.xml validates
If it does not validate, you will see what XSD rule is violated, together with the line where that happened eg:
410460: Schemas validity error : Element 'CCR5': [facet 'enumeration'] The value '' is not an element of the set {'DD', 'WW', 'DW'}.
Viewing large files
XML files tend to be large. For quick visual inspection and search you may use Glogg (windows)
#
File names
Registries with data on stem cell donors and cord blood units should separate these two data sets and provide two files: one for stem cell donors, and one for cord blood units. Data of stem cell donors and cord blood units should not be combined in one file. In the filename the distinction between donor data and cord blood unit data is made clear.
The first part of the filename is "ION-" (without the quotes) followed by the ion number and either a "D-" for donors (without the quotes) or a "C-" (without the quotes) for cold blood units. This <ION> should be similar to the one provided in the field <POOL>. The extension of the file is ".XML". Using this naming convention the name of the Austrian cord blood registry is: ION-2614-C.XML and the name of the German donor registry is: ION-6939-D.XML
After encryption, the file should follow almost the same name convention as for the xml file name, but then xml is replaced to pgp. So the first part of the GPG-filename is identical to the XML-filename. The GPG software will either add a second extension ".PGP", or replace the ".XML" extension of the data file with the ".PGP" extension. As an example, the file name of an encrypted Austrian cord blood file would then be: "ION-2614-C.PGP" (without the quotes).
If you are listing organisation and are also sending data from other listing organisations (with ION), you can provide the inventory of different POOL IDs together in one file. However, you should not combine donor and CBU data together. For the file naming, please always use the ION from the organisation that is sending the data.
#
How to compress your XML file with ZIP
When your file is larger then 200Mb, you have to compress your file by using ZIP. If your file is smaller, you are also encouraged to compress your file, because the time to upload your file will be reduced.
Please find below some methods to compress your file with ZIP when you are using any of the operating systems Windows, OS, or Linux/unix.
Please note: When you are using another method to compress your file, like tar, we cannot decompress your file during processing and we have to reject the file.
Creating a compressed zip file in Windows
- Click to highlight the file that you need to zip. Please note: WMDA can only accept your file when the zip file contains 1 file.
- Right-click the file and select Send to > Compressed (zipped) Folder.
- Windows will create the zip file and will position the cursor where you can choose a unique name for the file.
It is also possible to first create your ZIP folder and then drag the file to your zip folder.
Creating a compressed zip file in OS X
- Open a new Finder window and navigate to the file.
- Click to highlight the file that you need to zip.
- Select File > Compress from the pull-down menu. Sometimes you can also click with your right mouse button on the file and use the quick menu.
- Finder will compress the selected file and will create the zip file with the same same as your file but with the extension .zip.
Creating a compressed zip file in Linux
- Open a terminal session and navigate to the location of your file
- To view a listing of directory contents, enter the following command: ls
- Note the files to be zipped.
- Create the zip file by entering the command: zip {.zip-filename} {filename-to-compress} (e.g. zip ION-1234-D.zip ION-1234-D.xml)
#
How to encrypt your XML file
WMDA will only accept pgp encrypted XML files for data upload. We will now describe how you can encrypt your xml file. If you have a very large file, you should first compress your file with ZIP before you encrypted your file. Please see the picture below for a schematic representation of the encryption en decryption process.
Encryption is performed by the organisation who is sending data to WMDA; decryption is performed by WMDA to be able to process and validate the data in your file.
For this encryption, you should use the public key.
This is the new public key: public key
You may also fetch the key from http://pool.sks-keyservers.net/pks/lookup?op=get&search=0xC44E0E7A736E374E
Please note: This key is different from the key that you used to encrypt your DOT20 file.
STEP 1: Encryption program
The DOT20 file also needed to be encrypted. The procedure is actually the same, but you now have to use the new public key.
Currently, there are several different software packages that you can use to encrypt and decrypt your files. It depends of course also of your operating system which programs you can use.
Here are some examples:
Windows: Kleopatra, PGP Tool
OS (MAC): GPG Suite
Linux/unix: GnuPG
GnuPG is a complete and free implementation of the OpenPGP standard as defined by RFC4880 (also known as PGP ). GnuPG allows to encrypt and sign your data and communication, features a versatile key management system as well as access modules for all kinds of public key directories. GnuPG, also known as GPG , is a command line tool with features for easy integration with other applications. A wealth of frontend applications and libraries are available. GnuPG is Free Software (meaning that it respects your freedom). It can be freely used, modified and distributed under the terms of the GNU General Public License . For installation on your Linux/Unix machine, please visit the following page for HowTos: https://www.gnupg.org/documentation/howtos.html
STEP 2: Import the public key
After installing your preferred program, you have to import the public key.
- First download the public key to your computer. It is important that you save the file with extension asc (key.asc)
- Open your encryption program and look for something like import (PGP) key or import certificate. Click on this and then you have to upload the file with the WMDA public key and save the key.
- If you might get an error with importing the key, you can try to remove the text “Version: GnuPG v2” from the file and try to import the file again.
For Linux/Unix, importing of the key in your gpg keyring can be done by using the following command:
$ gpg --import {public_key_file}Alternatively, instead of saving this file and importing the key, you may look it up at a keyserver.
For example, if you use Kleopatra, use CTRL-SHIFT-I and search for 0xC44E0E7A736E374E
Getting the key : http://pool.sks-keyservers.net/pks/lookup?search=0xC44E0E7A736E374E
If you use gpg from the commandline, use gpg --recv-keys --keyserver pool.sks-keyservers.net --recv 0xC44E0E7A736E374E
STEP 3: Encrypt XML or ZIP file with the public key
Next step is to use the public key that you just imported into your encryption program to encrypt your XML or zipped XML file.
- In your encryption program, go to the function called encrypt or encrypt files.
- A windows with all your files will open. Look up the file you would like to encrypt.
- Following the steps in your program and make sure you choose the public key to encrypt your file
- Some programs work together with your file exploring program like Finder for Mac or Explorer for windows. If this is the case, go to your file look-up program and look for your file.
- Select your file and click on the right mouse button. A quick menu will become visible. Look for something with encryption or GPG or with a MAC it is probably under Services. This depends on the program you installed on your computer. Click on that and follow the instructions on your screen.
- Make sure you choose the public key to encrypt your file.
For Linux/Unix, encryption of your file can be done by using the following command:
$ gpg --encrypt --recipient ID {filename_to_be_encrypted}
where ID is replaced with that key's ID
The short version of the above command is:
$ gpg -e -r ID {filename_to_be_encrypted}
In either case, a file is created with the same name, plus an additional .gpg file extension added to the end. Thus, if your file is ION-1234-D.xml, you will create an encrypted copy of the file named ION-1234-D.xml.gpg.
Please note: Do not sign your file. This will result in rejection of your file during processing.
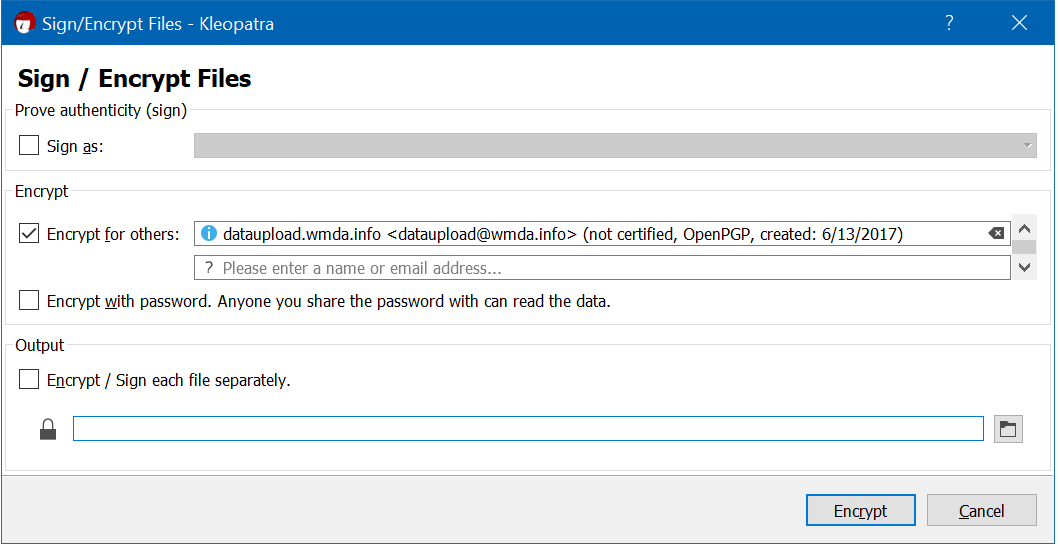
An example of the settings you should use when using Kleopatra (Windows) :
#
Explanation of Errors and Warnings in your processing report
INFORMATION
We are currently working on the new errors and warning you might find in your processing report. The errors and warnings shown below are mainly based on the old processing reports and are amended to our best knowledge at this moment. More information will follow soon.
After submitting a file you will receive a processing report in your data upload service account. This document describes explanations of warnings or errors as you may find them in the processing report. In the explanations below you find references to various fields from the file format for data delivery. For details on these field names and the file format look in the tabs 'XML file' and 'File name' of this page.
The ID of the donor or CBU will be displayed as well. This helps you find the problem line in the file you have sent, and hopefully helps you correct the problem.
Business validation rules applied
WMDA has additional business validation rules in place to ensure that even though the data supplied on a field level might be correct they need to have passed the validation rules applied sometimes on multiple fields to ensure correct data is being added to the GCD database. As part of providing us the XML your organisation should also perform these checks to ensure the validity of the data you are providing.
Business validation rules in excel file
File Errors
FileError: Empty file or file without data.
Explanation: When the size of the received file is zero bytes, or no data could be read from the received file, this error is returned.
Record Errors
- RecordError: No ID specified: Explanation: The ID or GRID field should be given, and should not be blank.
- RecordError: Duplicate donor/CBU ID found: Explanation: Every donor or cord blood unit should be listed only once. During processing of your file, a donor or cord blood ID is listed more than once, only the first occurrence has been accepted. The second occurrence XXXXX has been rejected.
- RecordError: Invalid GRID ID specified: Explanation: The global registration identified provided is invalid. PLEASE NOTE: GRID format allowed is: XXXX XXXX XXXX XXXX XXX. Also only upper case letter and numbers are allowed.
- RecordError: Duplicate GRID ID found: Explanation: Every donor or cord blood unit should be listed only once. During processing of your file, a GRID ID is listed more than once, only the first occurrence has been accepted. The second occurrence XXXXX has been rejected.
- RecordError: Invalid ION & GRID specified: Explanation: The GRID ID and Listing organisation (ION) ID are contradictory.
- RecordError: "Collected Volume before processing" (VOL) has an incorrect value: Explanation: The "Volume Collected" (VOL) should be no less than 10, or more than 400 milliliters (ml).
- RecordError: "Total Nucleated Cells" (TNC) has an incorrect value: Explanation: The "Total Nucleated Cells" should be no less than 10, or more than 999 (10^7).
- RecordError: "Collected number of CD34+ cells" (CD34PC) has an incorrect value: Explanation: The value provided for the CD34+ cell count is not a numeric value with decimal point in units of 10^6.
- RecordError: "Collected number of mononuclear cells" (MNC_FRZN) has an incorrect value: Explanation: The value provided for the mononucleated cell count is rounded number of mononuclear cells in the units of 10^7.
- RecordError: Duplicate donor/CBU ID found: Explanation: Every donor or cord blood unit should be listed only once. If during processing of a file, a donor or cord blood ID is listed more than once, only the first occurrence is accepted, the second occurrence generates this error.
- RecordError: Invalid date format for field 'field name' given: Explanation:This error may be returned for date fields. The format for dates field should be in the format YYYY-MM-DD.
- RecordError: Donor is either too young or too old: Explanation:The age limits of donors are set by the WMDA. Donor age outside range of 18-60 years are rejected.
- RecordError: Invalid Gender value found (not "M" or "F"): Explanation: Gender of donors other than "M" (for Male) or "F" (for Female) are reported as an error.
- RecordError: Invalid CMV Status value found: Explanation: The CMV status provided is not one of the possible values for this field.
- RecordError: BANK_MANUF_ID not provided: Explanation: The BANK_MANUF_ID allows the Search report to indicate that the Cord blood bank unit is accredited. If you do not provide this ID the search report will not be able to indicate the cord blood bank unit as being accredited or not.
HLA/DNA Related Errors
HLA/DNA-ERROR: Invalid allele value X found for DNA-"Allele".
Example: <HLA><DRB4><DNA><FIELD1>01:01/</FIELD1><FIELD2>value</FIELD2></DNA></DRB4></HLA>
Explanation: The value for DRB4 contains a slash (/) which is invalid. If such a problem is found the allele values are blanked and processing of the record continues. So, this is a warning, and not an error that requires the record to be rejected!Another examples might be an C*04:AVK (AVK is assigned bij the NMDP as 01/02/03/04/05/06) which is not valid since the C*04:02 is not assigned.
HLA/DNA-ERROR: Invalid DNA string found: "some string"
Example: For HLA A, the following value is given: 01:01/01:02/01:03/01:04.
Explanation: The DNA string given A*01:01/01:02/01:03/01:04 is not valid since the A*01:04 does not exist. Another reported problem may be that a ambiguity in the format of A*01:01/02/03 is invalid.
HLA/DNA-ERROR: Invalid HLA antigen "some value" found for field "field name".
Example: Serological HLA A30/3 is given in the file.
Explanation: The antigen or search determinant value "30/3" for HLA-A in this example is invalid.
HLA/DNA-ERROR: Incomplete typing found: HLA-A and HLA-B are required.
Explanation: At least one HLA-A and -B antigen or serological value should be available to be able to match the record. If no HLA-A or -B values (either on DNA level or on serological level) are available the record is rejected.
HLA/DNA-ERROR: "DNA allele values" does not match "serology". Equivalents for DNA alleles are ...
Example: HLA-B*15:02,15:26N does not match serological HLA-B76,62. Equivalents for the DNA-B alleles are: 75(15)
Explanation: The serology and DNA values provided, are validated separately, but also matched. If there is no match between the provided serology and the provided DNA, the record is reported.
HLA/DNA-ERROR: Number of alleles for DRB3/4/5 is more than 2; DRB3/4/5 blanked.
Example: Values are given for DRB4 for FIELD 1 and FIELD 2 and also for DRB5 for FIELD 1
Explanation: Only two allele values are allowed for DRB3, DRB4 and DRB5 combined.
HLA/DNA-ERROR: DRB3 (or DRB4 or DRB5) does not match HLA-DR "values". DRB3 (or DRB4 or DRB5) blanked.
Example: Serology DR is 4 and 11; DRB5 is 01:XX
Explanation: DRB5 is associated with DR2, DR15(2), DR16(2) or DR1(rare). Likewise, DRB3 is associated with DR3, DR5, DR6, DR11(5), DR12(5), DR13(6), DR14(6), DR1403, DR1404, DR17(3), DR18(3); and DRB4 with DR4, DR7, DR9.
Cord blood bank IDs
In the XML file format we also defined two fields that are referring to cord blood banks: BANK_MANUF_ID and BANK_DISTRIB_ID.
- The BANK_MANUF_ID is the ID corresponding to the cord blood bank that manufactured the cord blood unit (CBU)
- The BANK_DISTRIB_ID is the ID corresponding to the cord blood bank that will distribute the CBU
Please find in the table below the ID you have to use for your cord blood banks. The number always consist of 4 digits and is not the same as your ION number. We expect that the BANK_MANUF_ID and the BANK_DISTRIB_ID will be the same for many CBUs.
PLEASE NOTE: EMDIS is using very similar fields, but here you need to use a different ID.
Benefits of this ID
The BANK_MANUF_ID is very important for us. These IDs allow us to identify if the CBU is from an accredited bank or not which will be displayed within the Search & Match Service and the search reports. If you are not providing this ID, we cannot link the CBU to the accreditation status of your cord blood bank registered in our administration. Especially when your cord blood bank is accredited, you might benefit from providing this ID as several transplant centres only want to select CBUs from accredited cord blood banks.
If the ID of your cord blood bank is not provided in the table below, please contact us by sending an email to support@bmdw.org and we will generate an ID and update this list. Please also check if the accreditation status is correct.
| ID | Organisation | Country | City | Accreditation |
| 1327 | Argentina National Cord Blood Bank | Argentina | Buenos Aires | AABB |
| 1372 | Murdoch Childrens Research Institute - BMDI Cord Blood Bank | Australia | Victoria | FACT |
| 1371 | Queensland Cord Blood Bank At The Mater | Australia | Brisbane | FACT |
| 1346 | Sydney Cord Blood Bank | Australia | Randwick | FACT |
| 1279 | Red Cross Blood Transfusion Service of Upper Austria | Austria | Linz | none |
| 1014 | Vita 34 Austria | Austria | Graz- Andritz | none |
| 1382 | Banque de Sang de Cordon, Cliniques Universitaires Saint Luc | Belgium | Brussels | FACT |
| 1265 | Cord Blood Bank UZ Leuven | Belgium | Leuven | FACT |
| 1385 | Institut Jules Bordet - ULB Cord Blood Bank | Belgium | Brussels | FACT |
| 1266 | Liege Cord Blood Bank | Belgium | Liege | FACT |
| 1381 | UZ Gent Cord Blood Bank | Belgium | Gent | FACT |
| 1363 | Banco da Rede BrasilCord-Banco de Sangue de Cordão Umbilical e Placentário do INCA | Brazil | Rio de Janeiro | none |
| 1397 | Bancos da Rede BrasilCord-BSCUP HEMOSC | Brazil | Florianópolis/SC | none |
| 1368 | Bancos da Rede BrasilCord-Banco de Sangue de Cordão Umbilical da Fundação Hemocentro de Brasília | Brazil | Brasília | none |
| 1399 | Bancos da Rede BrasilCord-Banco de Sangue de Cordão Umbilical e Placentário do HEMOCE | Brazil | FORTALEZA | none |
| 1858 | Bancos da Rede BrasilCord-Hospital Israelita Albert Einstein | Brazil | Sao Paulo | FACT and AABB |
| 1396 | Bancos da Rede BrasilCord-Sociedade Beneficente de Senhoras Hospital Sírio Libanes | Brazil | Sao Paulo | none |
| 1369 | Bancos da Rede-BrasilCord-Hospital de Clinicas de Porto Alegre | Brazil | Porto Alegre | none |
| 1393 | "Canadian Blood Services" Cord Blood Bank | Canada | Ottawa | AABB |
| 1351 | Hema-Quebec Public Cord Blood Bank | Canada | St-Laurent | FACT |
| 1021 | Victoria Angel Registry of Hope Public Cord Blood Bank | Canada | Markham (Ontario) | FACT and AABB |
| 1022 | Vidacel | Chile | Santiago | none |
| 1026 | Beijing Jiachenhong Bio-tech Co., Ltd | China | Beijing | AABB |
| 1240 | Guangzhou Cord Blood Bank | China | Guangzhou | AABB |
| 1027 | Shanghai Cord Blood Bank | China | Shanghai | none |
| 1213 | Banco Sangre Cordon Umbilical Bogota | Colombia | Bogota D.C. | none |
| 1350 | Public CBB Ana Rukavina | Croatia | Zagreb | none |
| 1389 | CYCORD PACBB | Cyprus | Nicosia | FACT |
| 1388 | Cord Blood Bank Czech Republic | Czech Republic | Prague | none |
| 1442 | Banque de Sang Placentaire du CHRU de Montpellier | France | Montpellier | FACT |
| 1377 | Besancon Cord Blood Bank | France | Besancon Cedex | FACT |
| 1379 | Bordeaux Cord Blood Bank | France | Bordeaux | FACT |
| 1357 | Bayerische Stammzellbank GmbH | Germany | Gauting | none |
| 1322 | DKMS Nabelschnurblutbank | Germany | Dresden | FACT |
| 1839 | Deutsche Stammzellspenderdatei Nabelschnurblut | Germany | Mannheim | FACT |
| 1886 | Norddeutsches Knochenmark- und Stammzellspenderregister | Germany | Hannover | none |
| 1887 | Universitaetsklinikum Erlangen Stammzellbank | Germany | Erlangen | none |
| 1039 | José Carreras Cord Blood Bank Düsseldorf-Universitätsklinikum Düsseldorf | Germany | Dusseldorf | FACT |
| 1042 | Hellenic Cord Blood Bank | Greece | Athens | FACT |
| 1043 | Thessaloniki Public Cord Blood Bank | Greece | Thessaloniki | none |
| 1408 | Hong Kong Red Cross Catherine Chow Cord Blood Bank | Hong Kong | Hong Kong | FACT |
| 1323 | Jeevan Stem Cell Bank | India | Chennai | none |
| 1859 | LifeCell International Pvt. Ltd. | India | Manesar, Gurgaon | AABB |
| 1048 | Royan Institute Cord Blood Bank | Iran | Tehran | none |
| 1954 | Iranian Cord Blood Bank | Iran | Tehran | none |
| 1380 | Bedomaich Chayi Cord Blood Bank | Israel | Jerusalem | none |
| 1384 | MDA Public Cord Blood Bank | Israel | Ramat-Gan | FACT |
| 1840 | Sheba Cord Blood Bank | Israel | Tel Hashomer | FACT and AABB |
| 1892 | Banca Del Sangue Cordonale Di Cagliari (Ccbb) | Italy | Cagliari | none |
| 1893 | Banca Del Sangue Del Cordone Ombelicale Di Sciacca | Italy | Sciacca | none |
| 1894 | Banca Del Sangue Di Cordone Ombelicale Di Verona | Italy | Verona | none |
| 1352 | Banca Di Tessuti E Cellule Regione Toscana Pisa Cord Blood Bank | Italy | Pisa | none |
| 1888 | Banca Sangue Placentare Regione Abruzzo Pecb | Italy | Pescara | FACT |
| 1211 | Banca del Sangue Placentare di Treviso (Treviso Cord Blood Bank) | Italy | Treviso | FACT |
| 1367 | CBB Roma Azienda Policlinico Umberto I | Italy | Rome | none |
| 1356 | Calabria Cord Blood Bank | Italy | Reggio Calabria | none |
| 1362 | Campania Cord Blood Bank | Italy | Napoli | none |
| 1354 | Emilia Romagna Cord Blood Bank | Italy | Bologna | FACT |
| 1387 | Florence Cord Blood Bank | Italy | Florence | none |
| 1890 | Liguria Cord Blood Bank | Italy | Genova | none |
| 1832 | Milano Cord Blood Bank | Italy | Milano | FACT |
| 1895 | Padova Cord Blood Bank | Italy | Padova | none |
| 1366 | Pavia Cord Blood Bank | Italy | Pavia | none |
| 1359 | Puglia Cord Blood Bank | Italy | San Giovanni Rotondo | none |
| 1360 | St. Eugenio Hospital | Italy | Rome | none |
| 1891 | Torino Cord Blood Bank | Italy | Torino | none |
| 1889 | Unicatt Cord Blood Bank | Italy | Rome | none |
| 1373 | Japanese Red Cross Cord Blood Bank | Japan | Tokyo | none |
| 1062 | Banco Central de Sangre; Centro Médico Nacional La Raza | Mexico | Mexico | none |
| 1061 | CNTS-National Center of Blood Transfusion | Mexico | Mexico | none |
| 1341 | Cord Blood Bank of Samara Regional Center of Family Planning and Reproduction | Russian Federation | Samara | none |
| 1843 | King Abdullah International Medical Research Center-Cord Blood Bank | Saudi Arabia | Riyadh | FACT |
| 1079 | Singapore Cord Blood Bank | Singapore | Singapore | FACT and AABB |
| 1082 | Slovenský register placentárnych krvotvorných buniek- Eurocord-Slovakia (SRPKB) | Slovakia | Bratislava | none |
| 1083 | Slovenia Donor + CB Bank ESPOK | Slovenia | Ljubljana | none |
| 1394 | Andalucia Cord Blood Bank (Malaga) | Spain | Málaga | FACT |
| 1390 | Barcelona CBB (Programa Concordia BST) | Spain | Barcelona | FACT |
| 1383 | Basque Country Cord Blood Bank | Spain | Galdakao | none |
| 1914 | Canarias Cord Blood Bank | Spain | Tenerife | none |
| 1911 | Galicia Cord Blood Bank | Spain | Santiago de Compostela | none |
| 1915 | Ivida Cord Blood Bank | Spain | Madrid | FACT |
| 1912 | Madrid Cord Blood Bank | Spain | Madrid | none |
| 1913 | Valencia Cord Blood Bank | Spain | Valencia | FACT |
| 1916 | VidaCord Cord Blood Bank | Spain | Madrid | none |
| 1342 | Swedish National Cord Blood Bank | Sweden | Gothenburg | FACT |
| 1375 | Cord Blood Bank Basel | Switzerland | Basel | none |
| 1392 | Geneva Cord Blood Bank | Switzerland | Geneva 14 | none |
| 1088 | Bionet Corporation | Taiwan | Taipei City | AABB |
| 1089 | Buddhist Tzu Chi Stem Cells Center - BTCSCC | Taiwan | ROC Hualien | none |
| 1090 | Healthbanks Biotech Co., Ltd | Taiwan | Taipei City | FACT and AABB |
| 1204 | Meribank Biotech Co., Ltd, | Taiwan | New Taipei City | none |
| 1406 | Sino Cell Technologies Ltd. | Taiwan | Taipei | none |
| 1091 | StemCyte Taiwan Cord Blood Bank | Taiwan | New Taipei City | FACT and AABB |
| 1365 | Thai National Cord Blood Bank Bangkok | Thailand | Bangkok | none |
| 1287 | Sanquin Cord Blood Bank | The Netherlands | Leiden | FACT |
| 1386 | Ankara University Cord Blood Bank | Turkey | Ankara | FACT |
| 1841 | Anthony Nolan Cord Blood Bank | United Kingdom | Nottingham | FACT |
| 1391 | NHS Cord Blood Bank London | United Kingdom | Bristol | FACT |
| 1854 | Precious Cells International | United Kingdom | London | none |
| 1343 | Bloodworks NW Cord Blood Services | United States of America | Seattle | AABB |
| 1358 | CHOC Cord Blood Bank | United States of America | Orange | FACT |
| 1221 | Cleveland Cord Blood Center | United States of America | Cleveland, Ohio | FACT and AABB |
| 1842 | Cord: Use Public Cord Blood Bank | United States of America | Orlando | FACT and AABB |
| 1374 | Duke University Medical Center - Carolinas Cord Blood Bank | United States of America | Durham | FACT |
| 1361 | GenCure: Texas Cord Blood Bank | United States of America | San Antonio | AABB |
| 1353 | ITxM Cord Blood service | United States of America | Rosemont | AABB |
| 1364 | JP McCarthy Cord Stem Cell Bank | United States of America | Detroit, MI | FACT |
| 1345 | LifeCord/LifeSouth Community Blood Centers | United States of America | Gainesville | FACT |
| 1268 | Lifeforce Cryobanks | United States of America | Altamonte Springs | AABB |
| 1370 | MD Anderson Cord Blood Bank | United States of America | Houston | FACT |
| 1355 | Michigan Blood Cord Blood Bank | United States of America | Grand Rapids | AABB |
| 1344 | New Jersey Cord Blood Bank | United States of America | Montvale | AABB |
| 1844 | San Diego Cord Blood Bank | United States of America | San Diego | AABB |
| 1349 | St. Louis Cord Blood Bank | United States of America | Saint Louis, Missouri | AABB |
| 1376 | University of Colorado Cord Blood Bank | United States of America | Aurora | AABB |
| 1831 | Celebration Stem Cell Centre | United States of America | Gilbert | AABB |
| 1101 | LifebankUSA-HLI Cellular Therapeutics | United States of America | Cedar Knolls | AABB |
| 1103 | National Cord Blood Program New York Blood Center | United States of America | New York | FACT |
| 1298 | StemCyte Inc. | United States of America | Baldwin Park | FACT and AABB |
| 1242 | Cord Blood Bank, Ho Chi Minh City | Vietnam | Ho Chi Minh City | none |